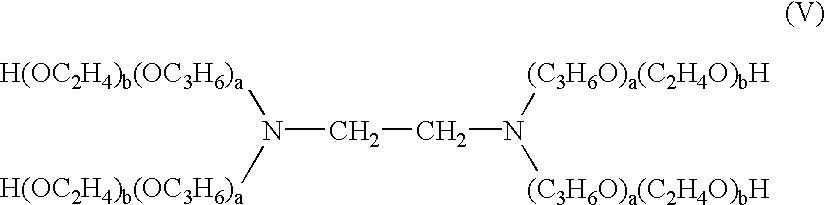Method and composition for inhibiting post-surgical adhesions
a post-surgical adhesion and composition technology, applied in the direction of drug compositions, powder delivery, medical preparations, etc., can solve the problems of reducing the effect of dextran intraperitoneally, preventing post-surgical adhesion formation, and reducing the effect of said solution on the separation of the uterine cavity walls
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
[0059] The following formation was prepared:
1 Ingredient Percent by Weight Poloxamer 407, NF 28.00 Pentoxifylline 0.40 Tromethamine (TRIS), USP 0.1091 Maleic Acid 0.1045 Sodium Hydroxide pellets, USP 0.0420 Sterile Water for Irrigation, USP 71.344
[0060] First, the water was weighed in a tared vessel. Second, the tromethamine, maleic acid and sodium hydroxide were added to the water and mixed for twenty minutes. The pH and osmolality of the solution were tested and found to be 7.6 and 39 mOsm / Kg, respectively. The solution was cooled to 6.degree. C. and the poloxamer was added to the solution over a two-hour period with constant stirring. The solution was stored under a nitrogen atmosphere at 4.degree. C. for fifteen hours to effect a reduction in the foam content of the solution. The solution was tested for pH, osmolality, and viscosity and the results were 7.4, 123 mOsm / kg and 360,000 centipoise at 30.degree. C., respectively. The solution was packaged into 30 cc serum vials, cappe...
example 3
Analytical Method
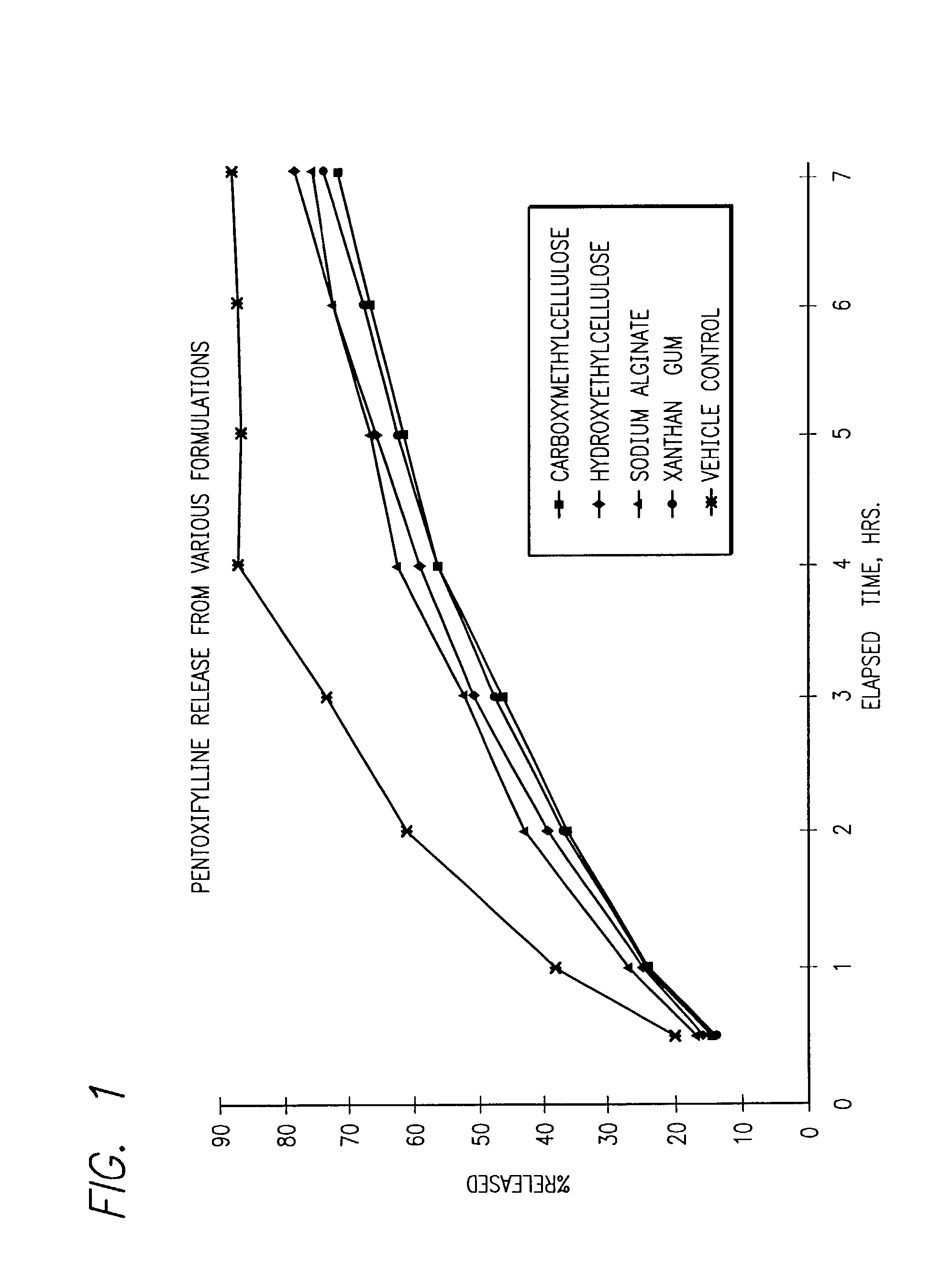
Measurement of Pentoxifylline Release from Various Formulations
[0061] Approximately 15 cm of SpectraPor regenerated cellulose dialysis tubing (6000-8000 molecular weight cut off, cat. #132650, Source: Spectrum Medical Industries, Inc.) is rinsed in distilled water to remove preservatives. One end is sealed with the appropriate sized closure.
[0062] 5 ml of the formulation to be tested was placed in the tubing and the other end sealed as close as possible to the sample. The tubing is placed in a beaker containing 95 ml of phosphate buffer (0.05 M KH.sub.2PO.sub.4+0.0391 M NaOH, pH 7.4) and a magnetic stirring bar. The solution was mixed on low speed at room temperature. One ml samples were withdrawn (via pipet) at each time point and diluted to an appropriate volume (either 10 or 25 ml) with water for UV absorbance measurements.
[0063] UV absorbance of the test solution is determined at 273 nm versus a water blank. A 100% release standard is prepared by diluting 5 ml o...
example 4
Surgical Trial on Prospective Study on Adhesions in the Rabbit Model
[0066] To assess the potential of poloxamer 407 with pentoxifylline to prevent or reduce adhesions, the following experiment was performed.
[0067] The following solution of of 14% poloxamer 407 with 1.2% pentoxifylline was prepared:
3 Ingredient Percent by Weight Poloxamer 407, NF 14.00 Pentoxifylline 1.20 Tromethamine (TRIS), USP 0.1091 Maleic Acid 0.1045 Sodium Hydroxide pellets, USP 0.0420 Sodium Chloride, USP 0.04 Sterile Water for Irrigation, USP 82.54
[0068] Water was weighed into a tared vessel. Pentoxifylline, tromethamine, maleic acid and sodium hydroxide were added to the water and mixed for twenty minutes. The pH and osmolality of the solution were tested and found to be 7.6 and 109 mOsm / Kg, respectively. The solution was cooled to 6.degree. C. and the poloxamer was added to the solution over a two-hour period with constant stirring. The solution was stored under a nitrogen atmosphere at 4.degree. C. for fif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com