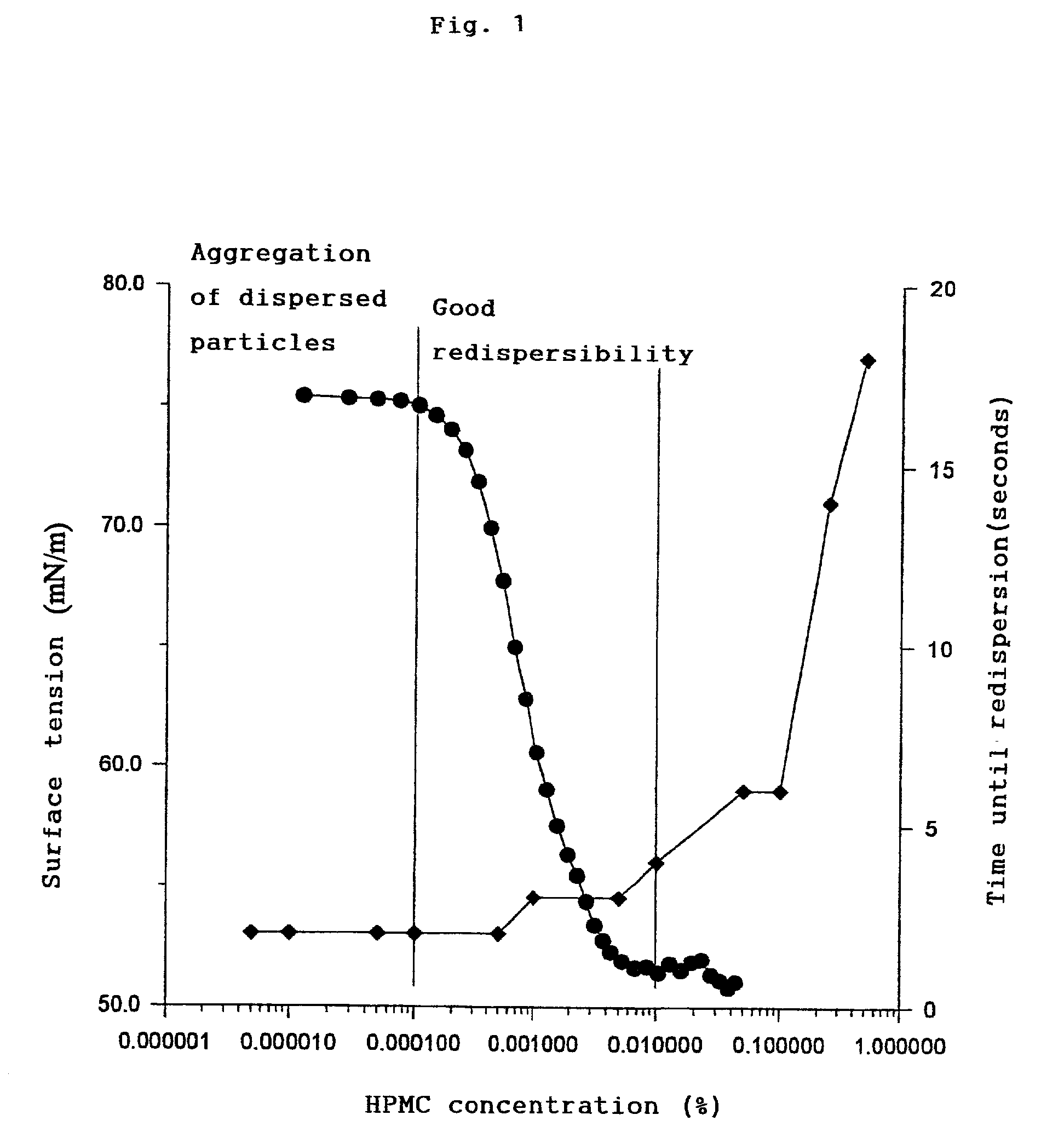
Aqueous suspension with good redispersibility
a technology of redispersibility and suspension, applied in the field of aqueous suspension with good redispersibility, can solve the problems of difficult to restore suspension uniform concentration, inability to employ the aqueous suspension form in many instances, and difficulty in restoring suspensions. uniformity, good redispersibility, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 5
5 Ophthalmic preparation Sulfamonomethoxine 0.1 g Hydroxypropylmethylcellulose 0.001 g Sodium acetate 0.1 g Benzalkonium chloride 0.005 g Sodium chloride 0.9 g 0.1 N Hydrochloric acid q.s. to make pH 5.0 Purified water q.s. to make 100 ml.
[0062] Hydroxypropylmethylcellulose was dissolved in about 80 ml of purified water by effecting dispersion with warming, followed by cooling to room temperature. Sodium chloride, sodium acetate and benzalkonium chloride were added and dissolution was effected. The pH was adjusted to 5 by adding hydrochloric acid. Sulfamonomethoxine was added and uniform suspension was effected by means of a mill. The whole amount was made 100 ml by adding purified water. A sulfamonomethoxine suspension ophthalmic preparation was thus prepared.
6 Example 6: Nasal drops Hydrocortisone acetate 0.1 g Hydroxypropylmethylcellulose 0.0008 g Sodium dihydrogen phosphate 0.1 g Methylparaben 0.026 g Propylparaben 0.014 g Concentrated glycerin 2.6 g 0.1 N Sodium hydroxide q.s....
example 7
7 Parenteral preparation (injection) Estradiol benzoate 5.0 g Hydroxypropylcellulose 0.03 g Chlorobutanol 0.3 g Sodium chloride 0.9 g Purified water q.s. to make 100 ml.
[0064] Chlorobutanol was dissolved in about 80 ml of purified water with warming. Hydroxypropylcellulose was dissolved in the solution by effecting dispersion with warming, followed by cooling to room temperature. Sodium chloride was added for dissolution, estradiol benzoate was added, and uniform suspension was effected using a homogenizer. The whole volume was made 100 ml by adding purified water. An estradiol benzoate suspension for parenteral administration was thus prepared.
8 Example 8: Preparation for oral administration Mefenamic acid 3.0 g Methylcellulose 0.01 g Sorbitol 20 g 5% Ethylparaben solution 1 ml Purified water q.s. to make 100 ml.
[0065] Methylcellulose was dissolved in about 50 ml of purified water by effecting dispersion with warming, followed by cooling to room temperature. Sorbitol and 5% ethylp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com