Method for the synthesis of a zero-valent metal micro- and nanoparticles in the presence of a noble metal
a nanoparticle and nanoparticle technology, applied in water treatment compounds, transportation and packaging, contaminated groundwater/leachate treatment, etc., can solve the problems of complex synthesis conditions, high cost of produced materials, and equipment required for correct execution of processes, and achieve simple operation conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
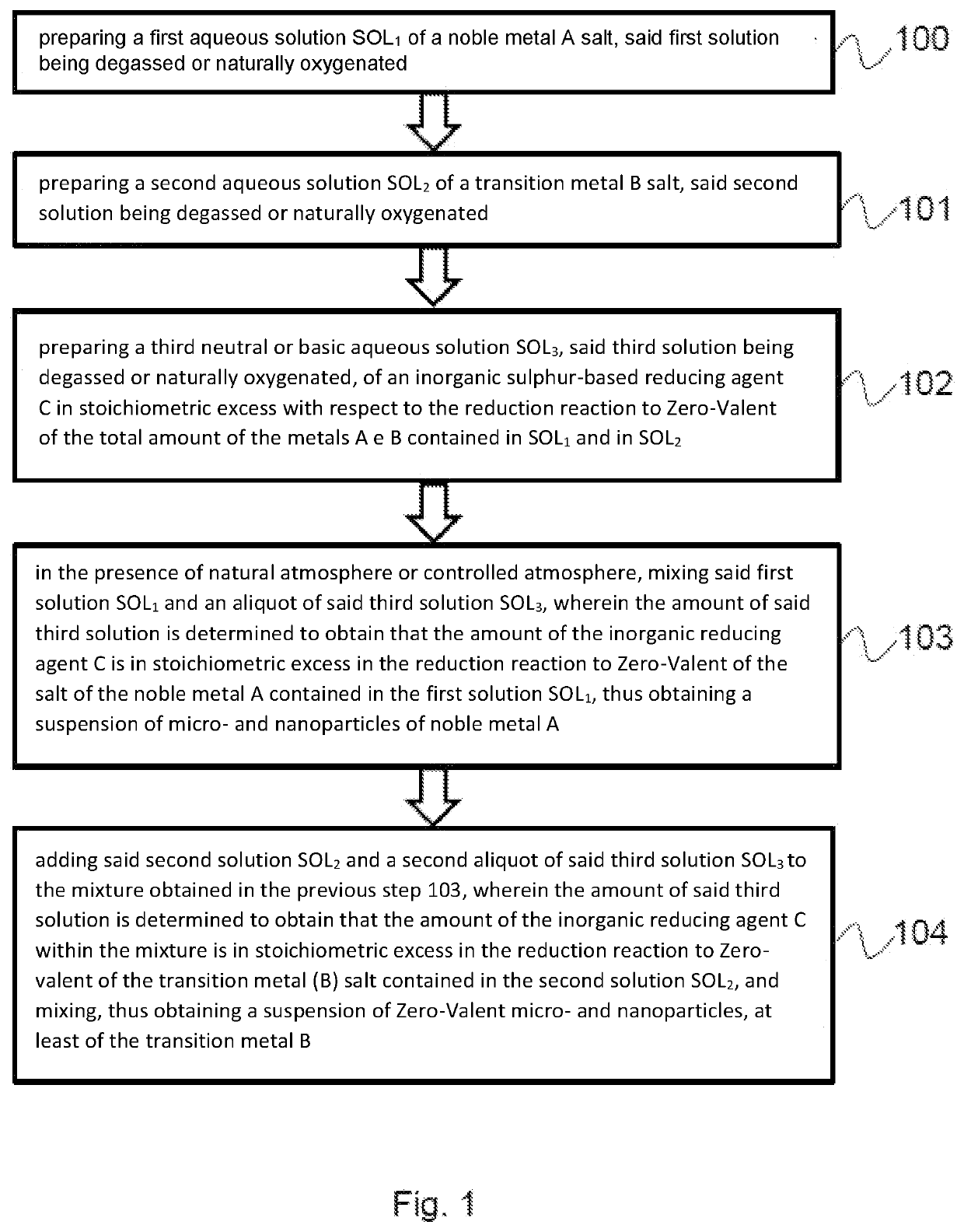
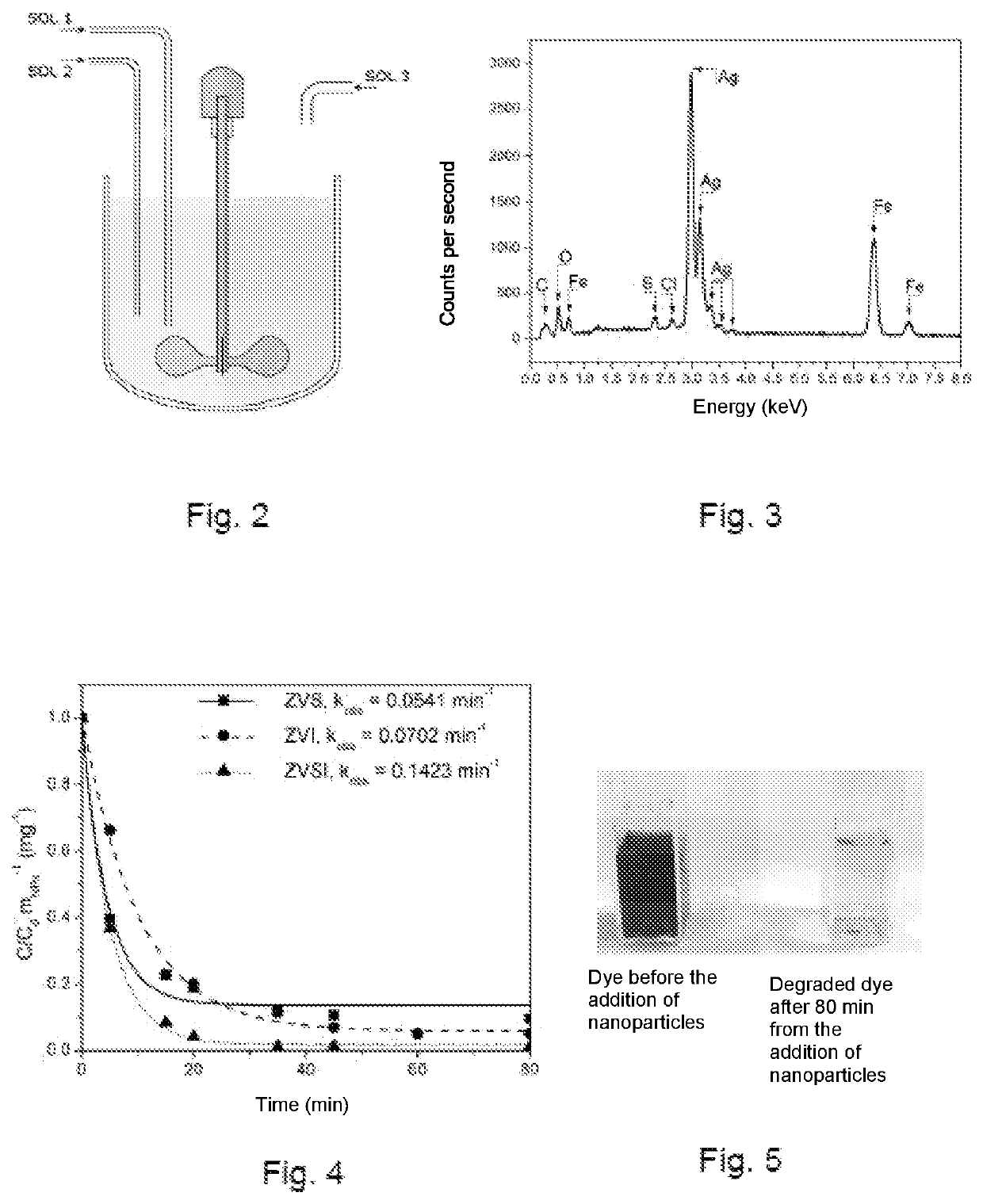
[0221]An example of an embodiment concerns the synthesis of Zero-Valent iron nanoparticles catalysed by pre-reduction of silver carried out by an inorganic reducing agent, the same or different from the one used to obtain the iron reduction. The synthesis of the bimetallic iron-silver nanoparticles was obtained following the present protocol:[0222]mixing 15 mL of a 2.4 mg / L AgNO3 solution with 5 mL of a sodium dithionite solution having a pH modified to 10 by adding an alkaline base: the reduction of Ag+ to Ag(0) and a lowering of the redox potential (pE) of the solution up to values close to the redox potential of the couple Ag+ / Ag(0) of about −0.8 V are obtained;[0223]adding 15 mL of a 10 mg / L solution of FeSO4 and a second aliquot of sodium dithionite (5 mL): the formation of black iron particles having an extremely magnetic character occurs;[0224]checking the amount of iron, as compared to stoichiometric products, it is possible to modify the magnetic properties and the product ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| mixing time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com