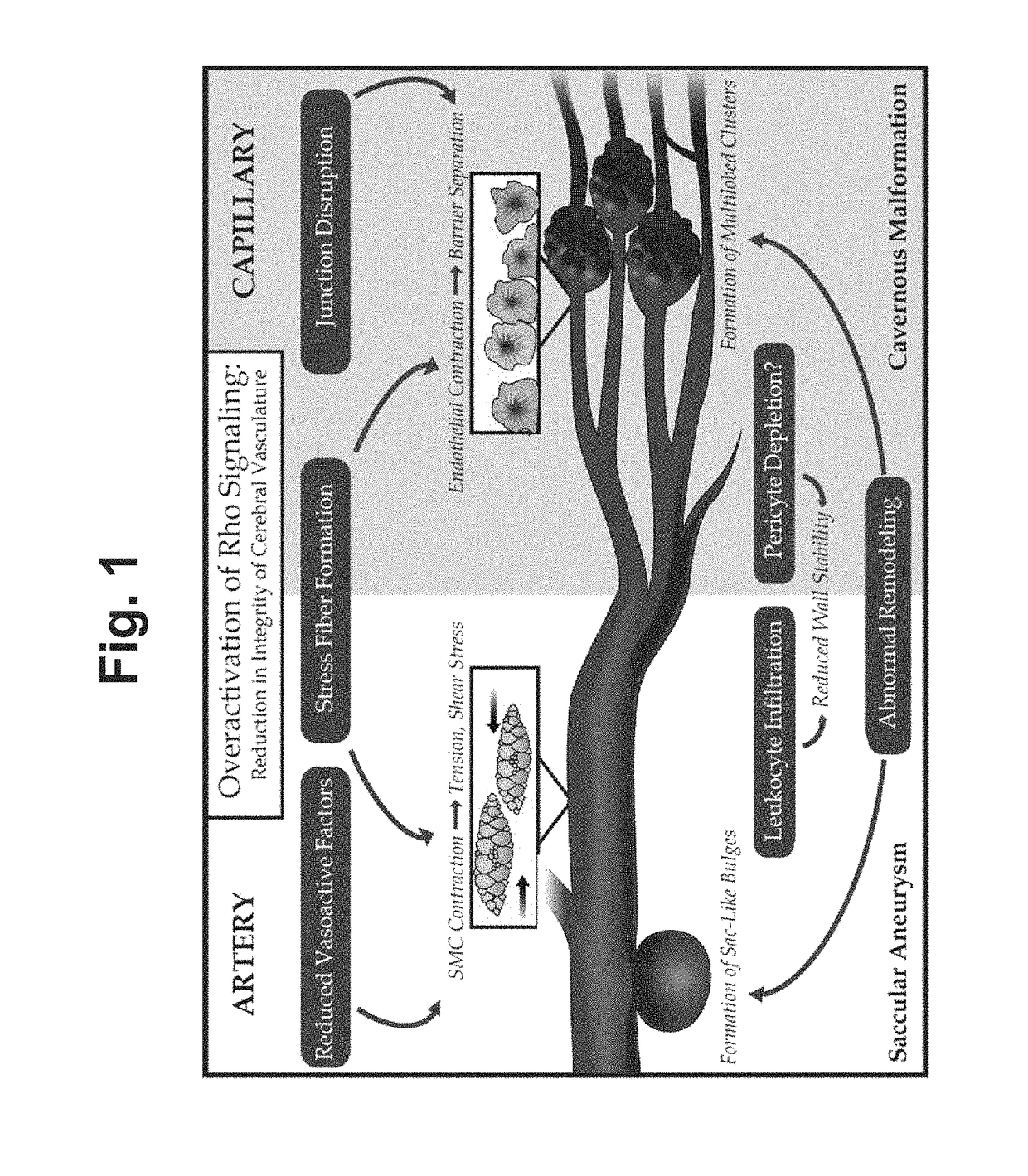
Treatment of cerebral cavernous malformations and cerebral aneurysms with rho kinase inhibitors
a technology of rho kinase inhibitor and cavernous malformation, which is applied in the field of medicine and neurology, can solve the problems of increasing the number of malformations, affecting the prophylaxis of cerebral aneurysms, and affecting the prophylaxis of cerebral cavernous malformations, so as to reduce the formation of certain types of cerebral aneurysms, stop the formation of ccm, and reduce the number of initial
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of an Enantiomer of BA-1049
[0080]The following scheme describes the synthesis of 50 mg to 100 mg of an enantiomer of BA-1049 (NT-000077) and includes a chiral synthesis method that enables the identification of the absolute configuration ((R) or (S)) of the molecule.
[0081]
[0082]To a solution of 1-Benzyloxycarbonyl-4-formylpiperidine (1) (2.0 g, 8.1 mM) in THF (20 mL) was added (S)-2-methylpropane-2-sulfinamide (1.0 g, 8.5 mM) followed by Ti(OiPr)4 (4.45 mL, 16.2 mM). The resulting solution was allowed to stir at room temperature (RT) for 18 hr, and then quenched with saturated aqueous NH4Cl and diluted with EtOAc. The mixture was filtered through a pad of celite, and washed with EtOAc. The layers were separated, and the organic phase was washed with brine, dried (MgSO4) and concentrated to give the crude residue which was purified by column chromatography (Isco 40 g) eluting with a gradient of Hexanes / EtOAc (70 / 30 to 30 / 60) to afford the desired imine 2 (2.2 g, 78%).
[0083]...
example 2
In Vitro Effect of Rho Kinase Inhibitors on Vascular Endothelial Cells
[0098]The following experiment demonstrates the ability of certain rho kinase inhibitors to restore key Wildtype (WT) features in vascular endothelial cells depleted of CCM proteins. These in vitro experiments are conducted in endothelial cell lines derived from CCM lesion biopsy in patients with Ccm1, Ccm2, or Ccm3 mutations, and in human microvascular endothelial cell line (HMVEC). The test rho kinase inhibitors are tested individually and in combination with the commercially available rho kinase inhibitor Fasudil.
[0099]Combinations of different ROCK inhibitors can tune the desired effect on endothelial cells after tube formation and on endothelial cells from patients with cavernous malformations. For example, by combining BA-1049 with Fasudil to give predicted ratio of ROCK I:ROCK II inhibition at ratios of 1:1, 1:10, or 10:1, the effects on the junctional process and stress fibers can be more finely modified. ...
example 3
Differential Tissue Expression of ROCK I and ROCK II in Neuronal Cell Lines
[0122]Two different neuronal cell lines (PC12 and NG108-15) were used to determine subcellular localization of ROCK I and ROCK II. The localization was studied with and without treatment for 24 hr with 10 μM of the rho kinase inhibitors BA-1049 (racemic mixture (Synthetica Fine Chemicals, Quebec, Canada), Fasudil (Calbiochem), or Y-27632 (Calbiochem).
A. Procedures
[0123]40,000 PC12 cells (ATCC, CRL-1721) were seeded on 8-well chamber, poly-L-lysine-coated (10 μg / mL) chamber slide and fixed. 30,000 NGIO8-15 cells (ATCC, HB-12317) were plated on 8-well chamber slide and fixed 16 hr-24 hr later.
[0124]After permeabilization, cells were incubated with specific polyclonal antibodies directed against ROCK I or ROCK II. FITC-conjugated rabbit IgG were used to visualize localization with the use of a fluorescent microscope. Results are representative of 2 independent experiments where at least 4 pictures were taken.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com