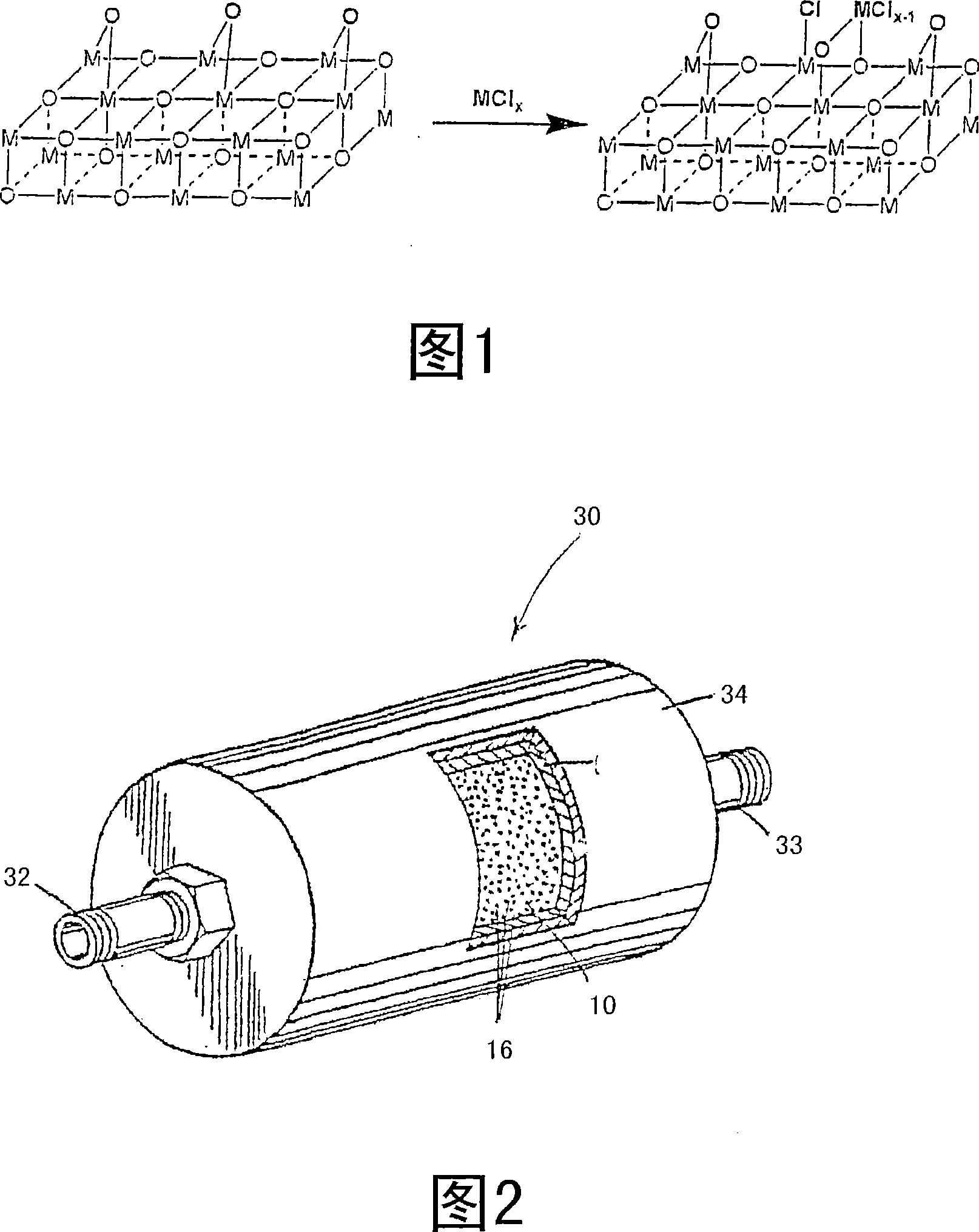
Removal of metal contaminants from ultra-high purity gases
An ultra-high-purity, pollutant technology, used in inert gas compounds, gas treatment, non-metallic elements, etc., can solve problems such as defective device performance, and achieve the effect of fewer defective products and increased product stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Purification of 10 Metal Contaminants from Copper Piping Systems
[0038] Individual pairs of silicon wafers were subjected to three different environments and subsequently analyzed for the presence of 10 selected metal contaminants with vapor phase decomposition using inductively coupled plasma mass spectrometry (VPD-ICP-MS). Each pair of wafers was flooded with nitrogen and stored in high-purity shipping boxes, triple-sealed with plastic bags and cleanroom tape until use.
[0039] The first pair of silicon wafers was checked for metal contamination using VPD-ICP-MS immediately after removal from the storage box.
[0040] A second pair of silicon wafers was placed in a Class 100 laminar flow hood. Pass high-purity nitrogen through hundreds of feet of copper piping. The gas is then passed through a gas purifier at a volumetric flow rate of less than 60 standard liters per minute (slm), wherein the purifying material is nickel / nickel oxide embedded on a si...
Embodiment 2
[0047] Example 2: Removal of Iron(III) Chloride from a Nitrogen Stream
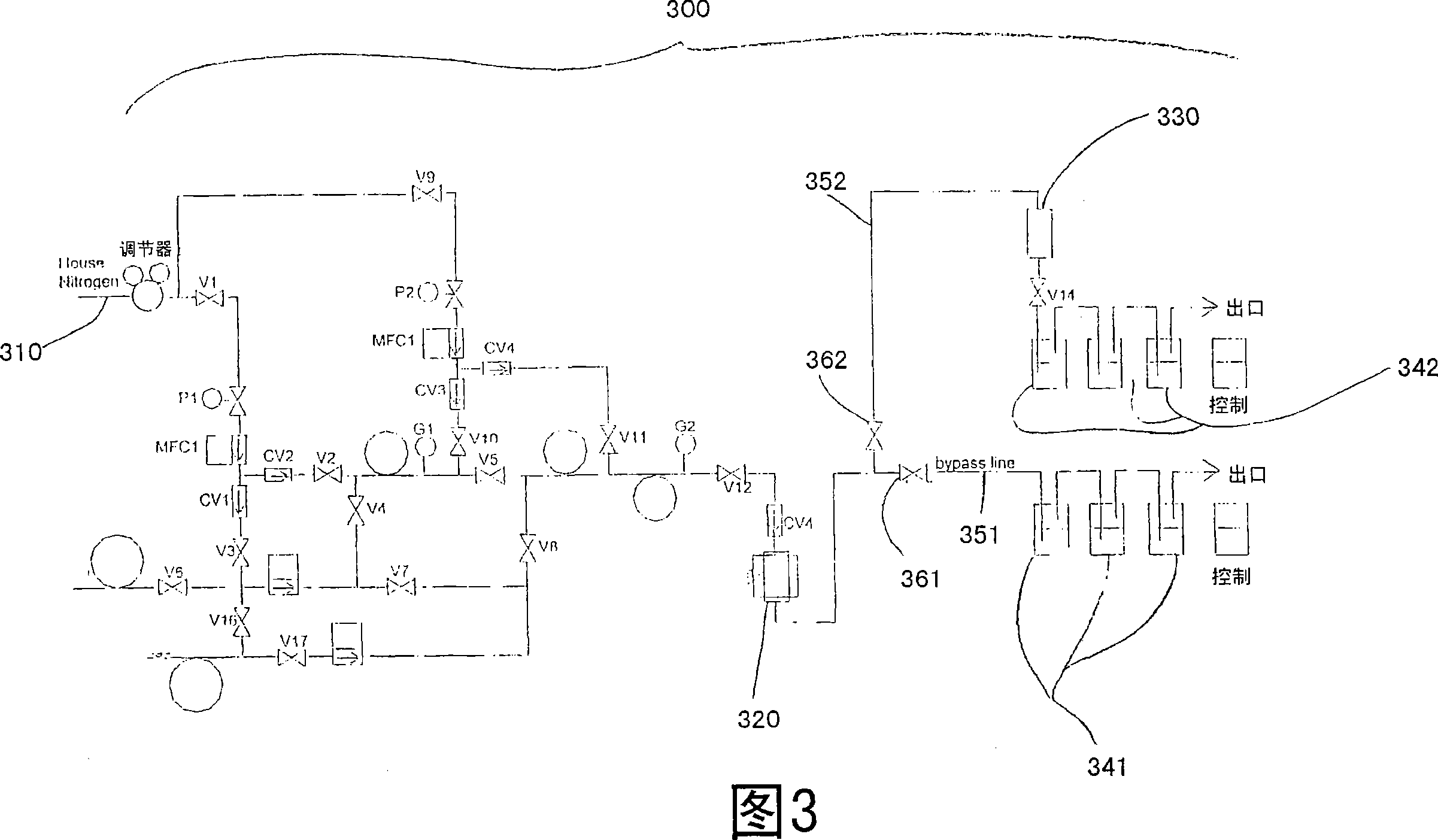
[0048] Conducting experiments to evaluate purification materials for removal of FeCl from nitrogen streams 3 Ability. Experiments were carried out using a test system 300 schematically shown in FIG. 3 .
[0049] Nitrogen is added to system 300 via line 310 . Fill approximately 40 mL of iron(III) chloride into enclosure 320, providing FeCl to be encased in a nitrogen test stream. 3 source. Wrap a heating mantle around the shell 320 and heat to 200°C to assist the FeCl 3 Bracketed in a stream of nitrogen.
[0050] Connected in parallel to the outlet line of the housing 320 are two sets 341 , 342 comprising three Teflon collection bottles respectively. Each Teflon collection bottle was pre-rinsed and filled with 2% dilute nitric acid solution to capture metal impurities. Each set of bottles is arranged in series. Valves 361, 362 respectively control the entrainment of FeCl 3 The nitrogen flow into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com