Batroxobin and its preparing process and specific coding gene
A technology encoding a gene and batroxobin, applied in botany equipment and methods, biochemical equipment and methods, genetic engineering, etc., can solve the problems of slow progress in molecular biology research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
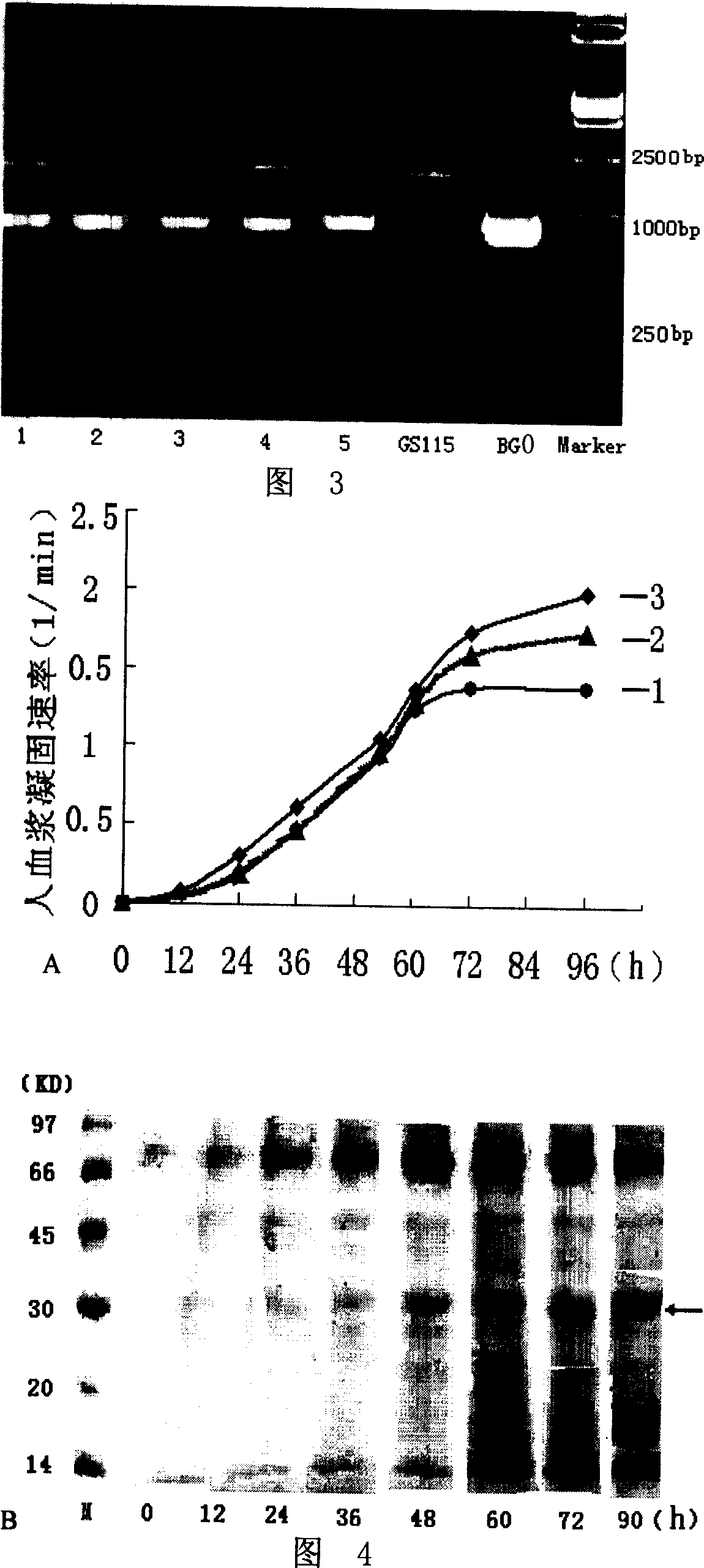
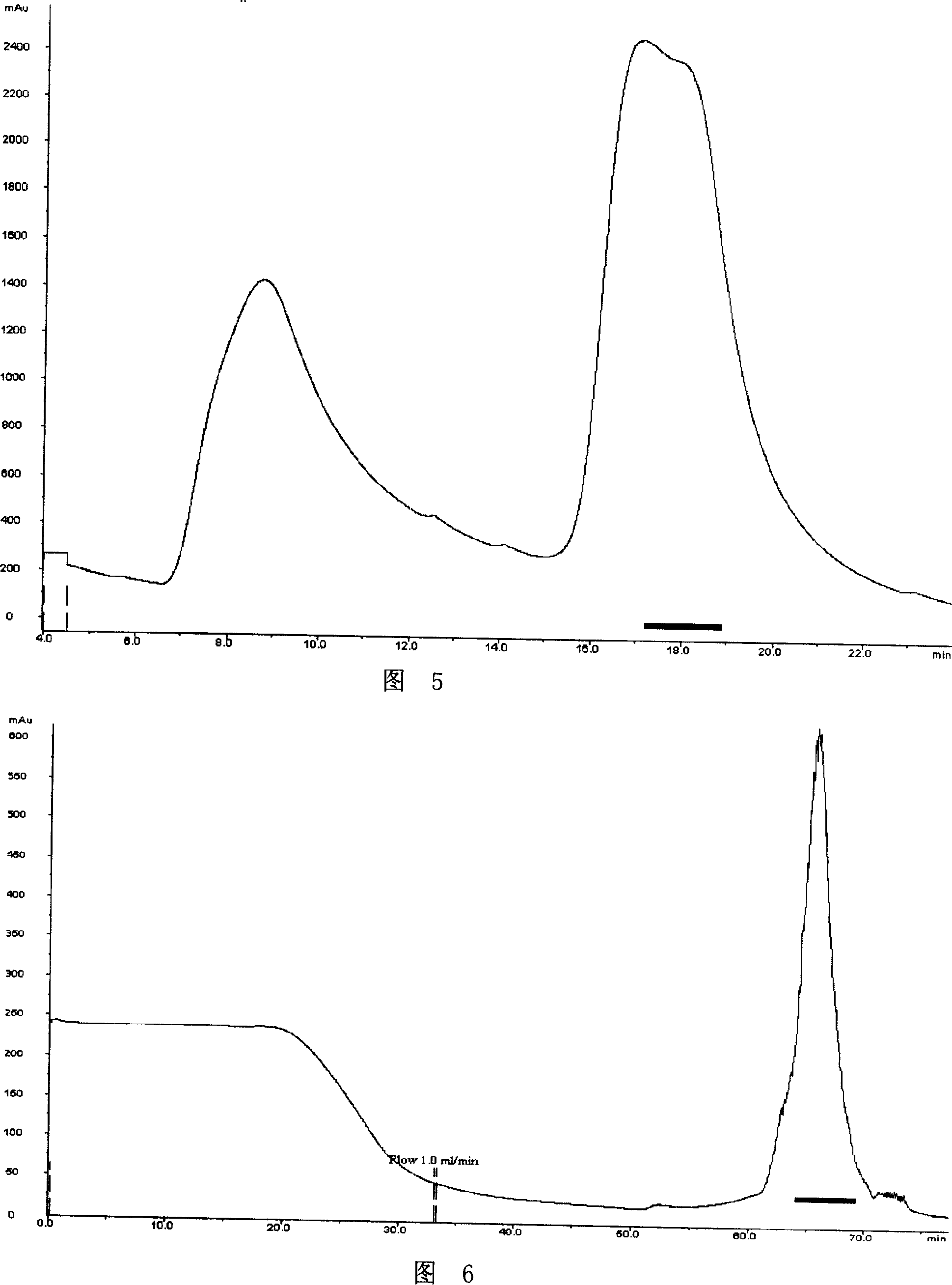
[0049] Embodiment 1, the synthesis of recombinant batroxobin gene BG0 and the detection of the effect of expressing batroxobin
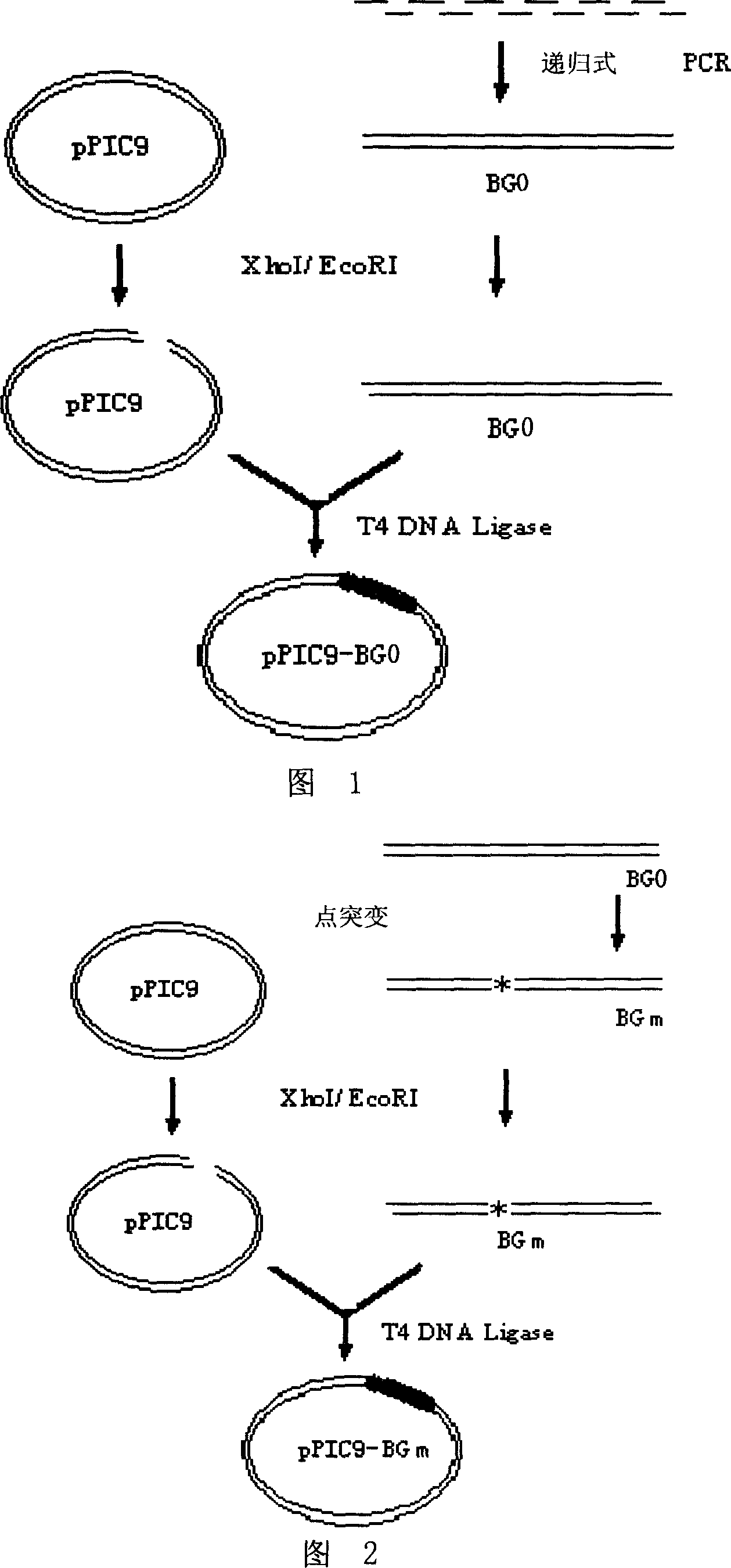
[0050] 1. Acquisition of recombinant batroxobin gene BG0 and construction of expression vector
[0051] 1) Acquisition of recombinant batroxobin gene BG0
[0052] According to the amino acid sequence data of batroxobin (GENBANK Accession Number is X12747) published in Genebank, the genetic codes that both yeast and CHO cells can be preferred were selected, and the full-length gene sequence of recombinant batroxobin was artificially synthesized (with Nucleotide sequence of sequence 2). In order to allow the target gene to be inserted into the pPIC9 vector, a cutting enzyme XhoI was added to the 5'-end of the Batroxobin Gene (BG) (GENBANK Accession Number is X12747 from the 1st to 693rd nucleotides at the 5' end). Add TAA stop codon and EcoRI cutting point at the 3′-end, and add the codon AAA corresponding to the KEX2 protease recognition sequence Ly...
Embodiment 2
[0091] Example 2. Synthesis of batroxobin gene (IBG0) containing IL-2 signal peptide gene and detection of the effect of batroxobin expression.
[0092] 1. Synthesis, sequencing and construction of expression vector of batroxobin gene (IBG0) containing IL-2 signal peptide gene
[0093] Batroxobin gene BG0 was used as template (sequence 2 in the sequence listing) and IL-2 signal peptide gene (from GENBANK number NM_000586 the 5' end 295-354 nucleotides) by splicing overlap extension PCR (SOE-PCR) sequence) to connect, the specific method is:
[0094] Using the IL-2 signal peptide gene (from GENBANK No. 295-354 nucleotide sequence at the 5' end of NM_000586) as a template, use IL25 primer: 5'-CTGGATCCACGATGTATAGGATGCAACTGCTG-3' and IL23 primer: 5'-AGCGCTGTTGGTGACCAG- 3' is the primer for PCR, PCR reaction system: IL-2 signal peptide gene (7.5uM) 0.1μL, IL25 primer (15uM) and IL23 primer (15uM) each 0.5μL, dNTP (each 2.5mM) 2μL, KOD DNA Polymerase (5U / μL)0.1μL, 10*KOD buffer 2μ...
Embodiment 3
[0106] Embodiment 3, research on the glycosylation of batroxobin protein expressed by BG0 gene
[0107] 1. Acquisition of batroxobin glycosylation site mutation genes (BGm1, BGm2) and construction of expression vectors (Figure 2)
[0108] The site-directed mutagenesis technique was used to mutate the BG0 gene, and Asn (from the 146th position of the amino terminal of sequence 1) was mutated into Gln (the motif of glycosylation is Asn-X-Thr), that is, the 5' end of sequence 2 in the sequence list The 448th-450th nucleotide (AAC) is mutated to CAA; the specific method is:
[0109] Using pPIC9-BG0 as a template, use a-Factor primer: 5′-TACTATTGCCAGCATTGCTGC-3′ and M13 primer: 5′- TTG GAACAGGTTAATGTTAGCACAG-3′( mutation codon ) as primers for PCR amplification, PCR reaction system: 0.1 μL (0.04ug) of pPIC9-BG0 plasmid, 0.5 μL of a-Factor primers (2.5 mM each) and 0.5 μL of M13 primers (15 uM each), 2 μL of dNTPs (2.5 mM each), KOD DNA Polymerase (5U / μL) 0.1μL, 10*KODbuffer 2μL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com