Method for removing impurity of tetrafluoroethylene
A technology for tetrafluoroethylene and impurities, which is applied in the field of removing impurities in tetrafluoroethylene, can solve the problems of affecting the quality of tetrafluoroethylene monomer, destroying the atmospheric ozone layer, and high operating energy consumption, achieving small investment, high operating flexibility, and operating costs. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
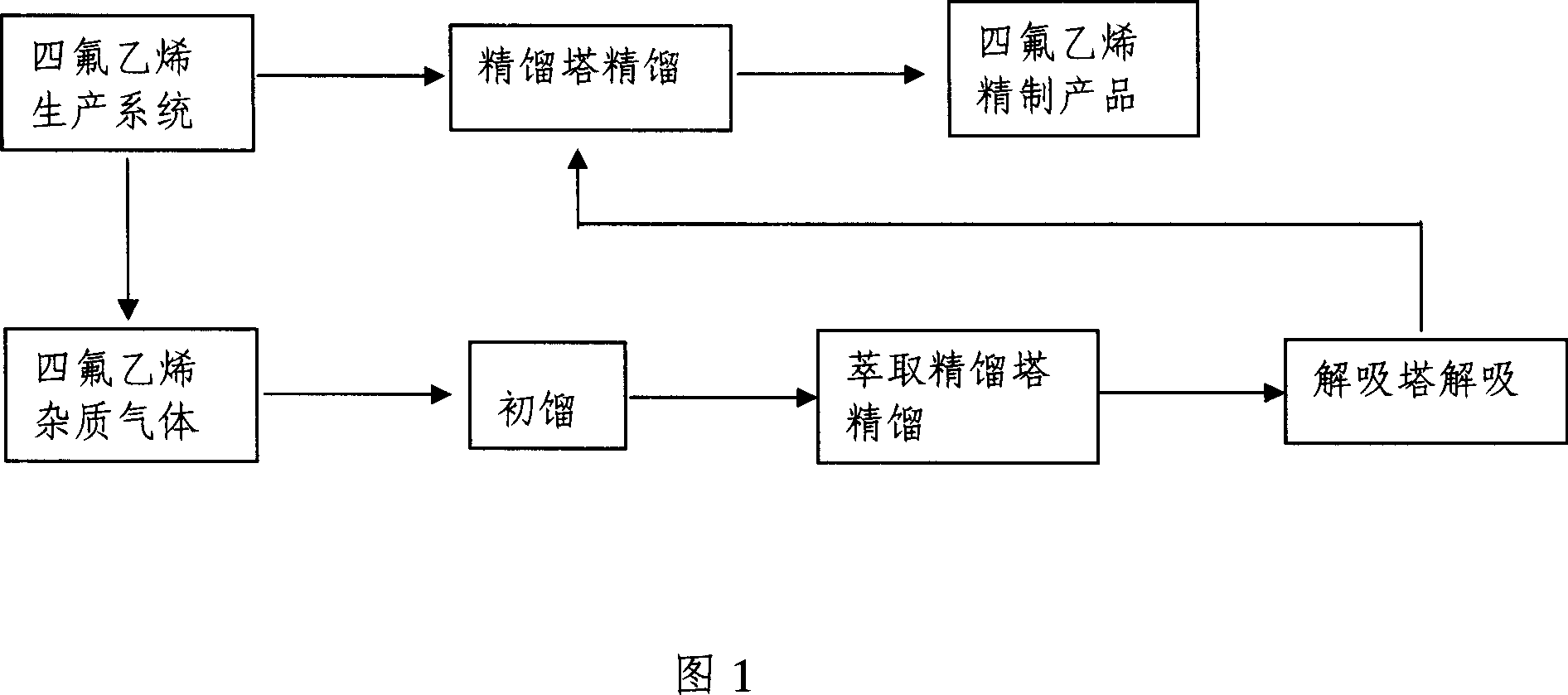
[0023] A method for removing impurities in tetrafluoroethylene. After initial distillation of tetrafluoroethylene gas containing impurities through a degassing tower, it enters an extractive distillation tower with a flow rate of 50kg / h for rectification. The extraction agent is methanol, and the extractive distillation tower The fluorine-containing impurity gas at the top of the tower is directly incinerated, and the components obtained from the distillation column are desorbed by the desorption tower and directly returned to the tetrafluoroethylene production system, and the tetrafluoroethylene monomer is obtained after rectification. The packing of the extractive distillation column is metal Interrox packing. During the extractive distillation, the liquid-gas ratio is 10:1. During the extractive distillation, the spray density of the extractive distillation column is 10m 3 / (m 2 .h), the spray temperature is -20°C. During the extractive distillation, the pressure of the ...
Embodiment 2
[0025] A method for removing impurities in tetrafluoroethylene. After initial distillation of tetrafluoroethylene gas containing impurities through a degassing tower, it enters an extractive distillation tower with a flow rate of 100kg / h for rectification. The extraction agent is acetone, and the extractive distillation tower The fluorine-containing impurity gas at the top of the tower is directly incinerated, and the components obtained from the distillation column are desorbed by the desorption tower and directly returned to the tetrafluoroethylene production system, and the tetrafluoroethylene monomer is obtained after rectification. The filler of the extractive distillation column is metal Pall ring particle filler. During the extractive distillation, the liquid-gas ratio is 5:1. During the extractive distillation, the spray density of the extractive distillation tower is 30m 3 / (m 2 .h), the spraying temperature is 0°C. During the extractive distillation, the pressure ...
Embodiment 3
[0027] A method for removing impurities in tetrafluoroethylene. After initial distillation of tetrafluoroethylene gas containing impurities through a degassing tower, it enters an extractive distillation tower with a flow rate of 150kg / h for rectification. The extraction agent is chloroform, and the extractive distillation tower The fluorine-containing impurity gas at the top of the tower is directly incinerated, and the components obtained from the distillation column are desorbed by the desorption tower and directly returned to the tetrafluoroethylene production system, and the tetrafluoroethylene monomer is obtained after rectification. The packing of the extractive distillation column is metal structured packing. During the extractive distillation, the liquid-gas ratio is 0.5:1. During the extractive distillation, the spray density of the extractive distillation tower is 50m 3 / (m 2 .h), the spraying temperature is 20°C. During the extractive distillation, the pressure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com