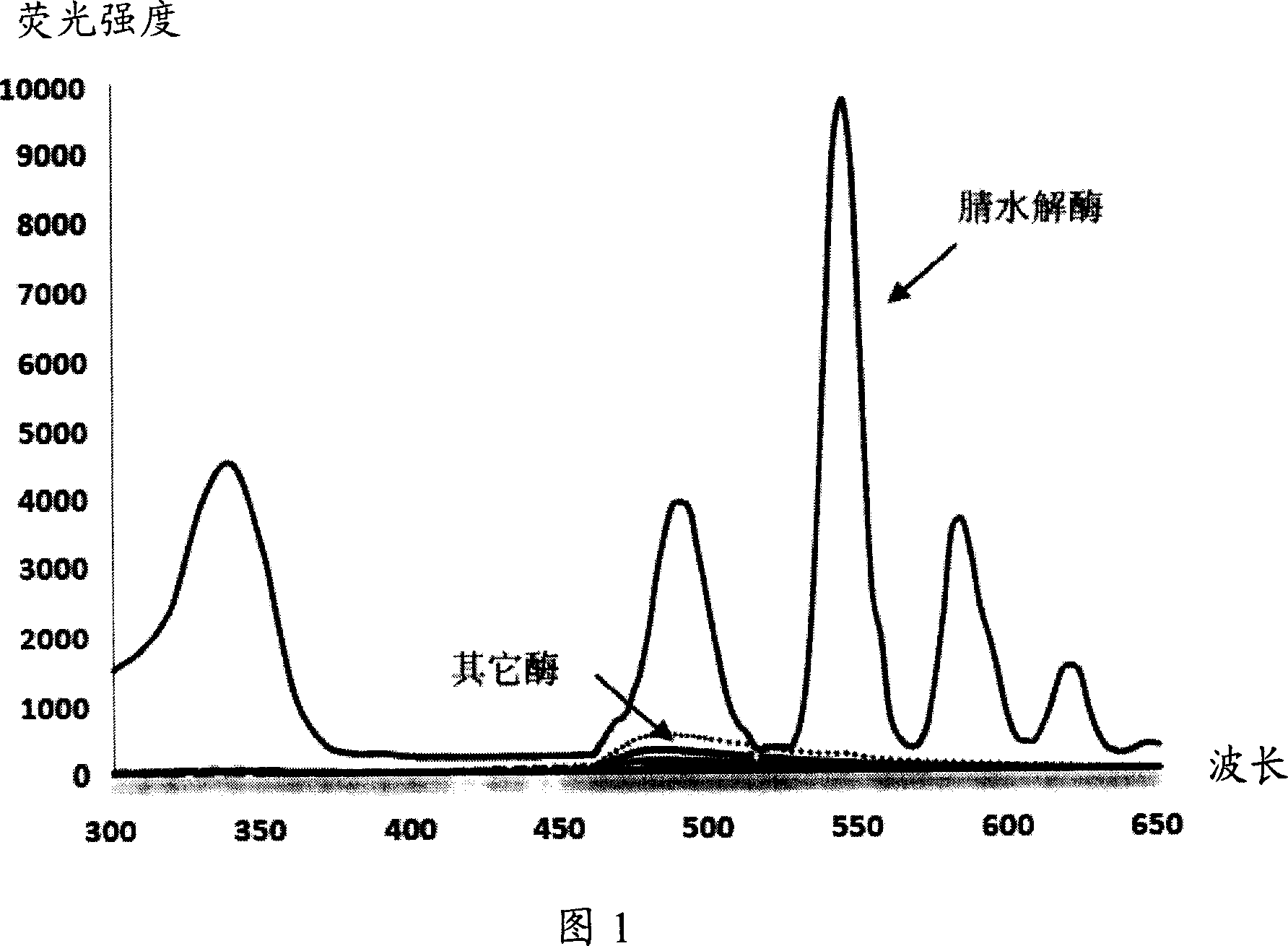
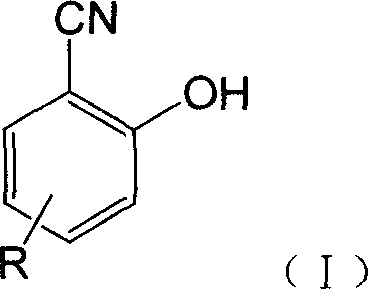
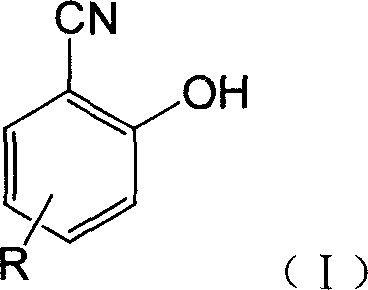
Fluorescent detecting method for nitrile hydrolitic enzyme activity
A nitrilase and fluorescence detection technology, which is applied in the field of fluorescence detection of nitrilase activity, can solve the problems of lack of fluorescent probe detection theory and methods, limit large-scale application, low sensitivity, etc., achieve stable properties, simple equipment, The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Get 3.90g of salicylaldehyde and 1.86g of hydroxylamine hydrochloride and add them into 0.066mol of sodium hydroxide solution. Then the reaction mixture was stirred and reacted in a water bath at 50°C for 2 hours to obtain a homogeneous solution of a color (yellow), and the pH was adjusted with 36% acetic acid until neutral, and it was placed in an ice bath, there will be Crystals were precipitated, then filtered, washed with water, and air-dried to obtain 2.50 g of the product (salicylaldoxime), with a yield of 67.8%. Then take 1.00 g of salicylaldoxime and 0.50 g of acetic anhydride into the reactor, heat, stir and reflux with an oil bath (160° C.), and react for 10 hours. After heating to reflux, the reaction solution is rotary distilled, unreacted acetic anhydride is distilled off, and 10% NaOH solution is added to the remaining liquid after distillation until the liquid is a clear liquid, and then an appropriate amount of absolute ethanol is added, Make the liquid...
Embodiment 2
[0030] In the NaOH solution (2mol / ml) of 30ml, successively add the 5-chloro salicylaldehyde of 2.00g and the hydroxylamine hydrochloride of 1.00g, (at this moment, the gained solution is not homogeneous, contains the solid of a small amount of particle wherein, so, in reaction A small amount of absolute ethanol was added dropwise in the reaction solution to dissolve a small amount of solid particles in the reaction solution) and the reaction mixture was stirred and reacted in a water bath at 50°C for 2 hours, and then 36% acetic acid was added to adjust the pH to neutral, with white The powdery solid is precipitated, and then the reactor is placed in an ice-water bath to stand still for a while, and then filtered, and the obtained white powdery solid is 5-chlorosalicylaldoxime, and the yield of this product is more than 90%. Then take 1.00g of 5-chloro salicylaldoxime and 0.50g of acetic anhydride into the reactor, heat, stir and reflux with an oil bath (160° C.), and react fo...
Embodiment 3
[0036] Take 1.00g of 5-fluorosalicylaldehyde and 0.50g of hydroxylamine hydrochloride, add successively to 30ml of NaOH (2mol / ml) solution, and stir the reaction mixture in a water bath at 50°C for 2 hours, then add 36% acetic acid to adjust When the pH is neutral, a white powdery solid is precipitated, and then the reactor is placed in an ice-water bath to stand still for a while, and then filtered, and the white powdery solid obtained is 5-fluorosalicylaldoxime, and the yield of this product is 95% . Then take 1.00g of 5-fluorosalicylaldoxime and 0.50g of acetic anhydride into the reactor, heat, stir and reflux with an oil bath (160° C.), and react for 8 hours. After heating to reflux, the reaction solution is rotary distilled, unreacted acetic anhydride is distilled off, and 10% NaOH solution is added to the remaining liquid after distillation until the liquid is a clear liquid, and then an appropriate amount of absolute ethanol is added, Make the liquid uniform (yellow in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com