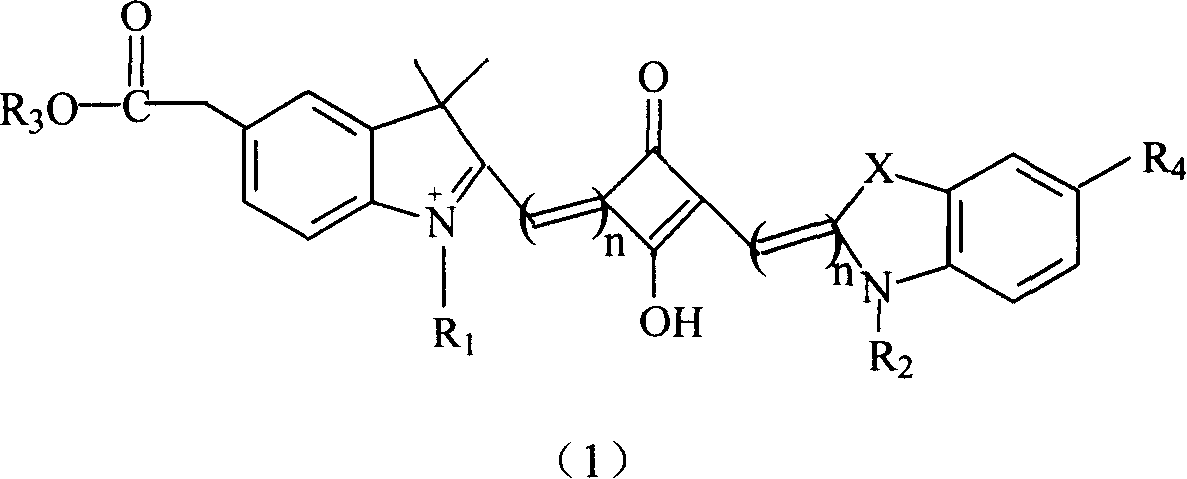
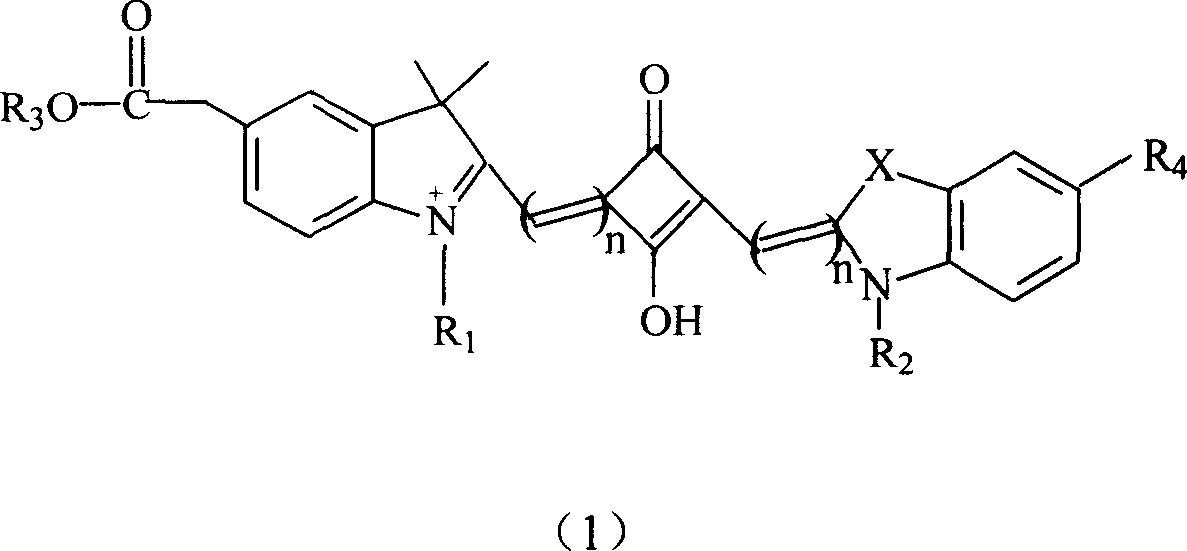
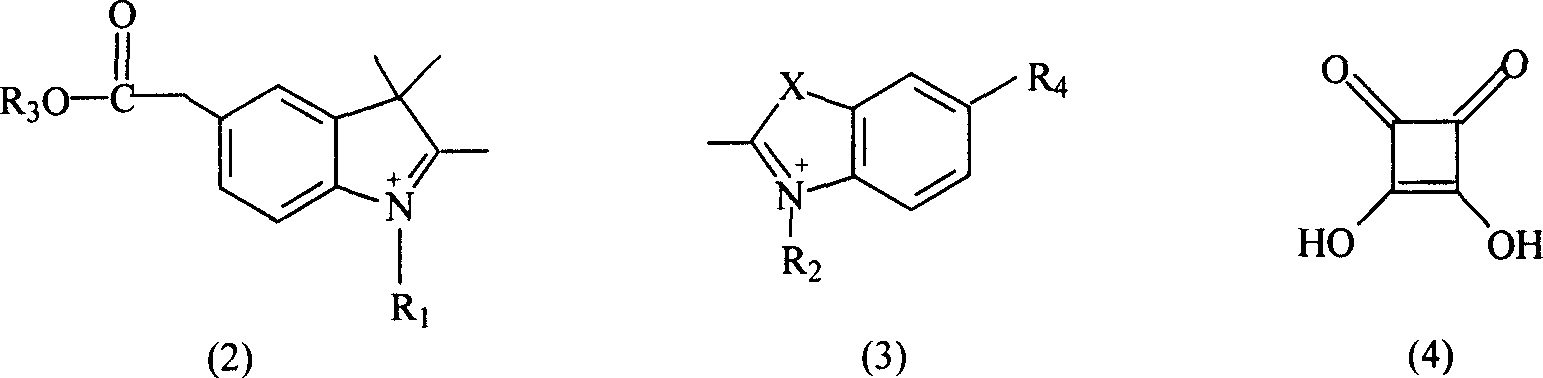
Soluble fluorescent cyanogen dye
A fluorescent cyanine and water-soluble technology, applied in the field of cyanine dyes, can solve problems such as poor photostability and affect analysis reliability, and achieve the effect of improving photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Synthesis of (2,3,3-trimethyl-3H-indol-5-yl)acetic acid (5)
[0022]
[0023] i) In a 250ml three-necked flask equipped with a mechanical stirring device, add p-aminophenylacetic acid (7.55g, 0.05mol), 20ml concentrated hydrochloric acid, 25ml ice water, slowly add sodium nitrite (3.45g, 0.05mol) dropwise at 0°C ) in 18ml of ice-water solution, keep the temperature at 0°C for 0.5 hour, and quickly filter to remove a small amount of precipitate. A solution of stannous chloride dihydrate (33.84 g, 0.15 mol) in 35 ml of hydrochloric acid was added dropwise to the filtrate at 0°C, stirred for 0.5 h to stop the reaction, and frozen overnight. The next day, the reaction solution was filtered, and the filter cake was washed successively with a mixture of saturated sodium chloride solution, petroleum ether and diethyl ether (2:1) to obtain 6.5 g of a pink solid with a yield of 64%. The product was directly obtained without purification. for the next reaction.
[0024] ii) ...
Embodiment 2
[0026] Synthesis of 1-ethyl-(2,3,3)-trimethylindole iodide (6)
[0027]
[0028] In a 25ml three-necked flask equipped with a reflux condenser and a drying tube, add 2,3,3-trimethylindole (1.6g, 0.01mol) and ethyl iodide (8ml, 0.1mol) under argon protection, and heat Reflux for 24 hours, add 50ml of acetone after cooling, a solid precipitates, and filter to obtain 1.9g of a khaki solid, with a crude yield of 60%. Recrystallization from ethanol gave a brownish-yellow solid, Mp.229°C (lit.Mp.226-228°C). 1 HNMR (D 2 O): δ7.76-7.60 (m, 4H, 4-H, 5-H, 6-H, 7-H), δ4.50 (q, 2H, J=7.6Hz, 8-H), δ1. 55 (s, 6H, 11-H, 12-H), δ1.53 (t, 3H, J=7.6Hz, 9-H).
Embodiment 3
[0030] Synthesis of 1-(δ-sulfonic acid butyl)-5-carboxymethyl-2,3,3-trimethylindole betaine (7)
[0031]
[0032] In a 25ml three-necked flask equipped with a reflux condenser and a drying tube, add 5-carboxymethyl-2,3,3-trimethylindole (0.65g, 0.003mol), 1,4-butane Sultone (1.23g, 0.09mol) and 2ml of o-dichlorobenzene were heated to 110°C, kept at this temperature for 5 hours, cooled to 60°C after the reaction, added 10ml of acetone and refluxed for one hour, cooled and filtered to obtain a brown powder The solid was 0.9 g, and the crude yield was 85%. Purple crystals were obtained by recrystallization from methanol and isopropanol (1:1.5). Mp.264-267°C (lit.Mp.287-290°C). 1 HNMR (D 2 O): δ7.75(d, 1H, J=8.3Hz, 7-H), δ7.65(s, 1H, 4-H), δ7.51(d, 1H, J=8.3Hz, 6-H ), δ4.50(t, 2H, J=7.6Hz, 11-H), δ3.84(s, 2H, 15-H), δ2.96(t, 2H, J=7.6Hz, 8-H) , δ2.14-2.07 (m, 2H, 10-H), δ1.90-1.85 (m, 2H, 9-H), δ1.55 (s, 6H, 13-H, 14-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com