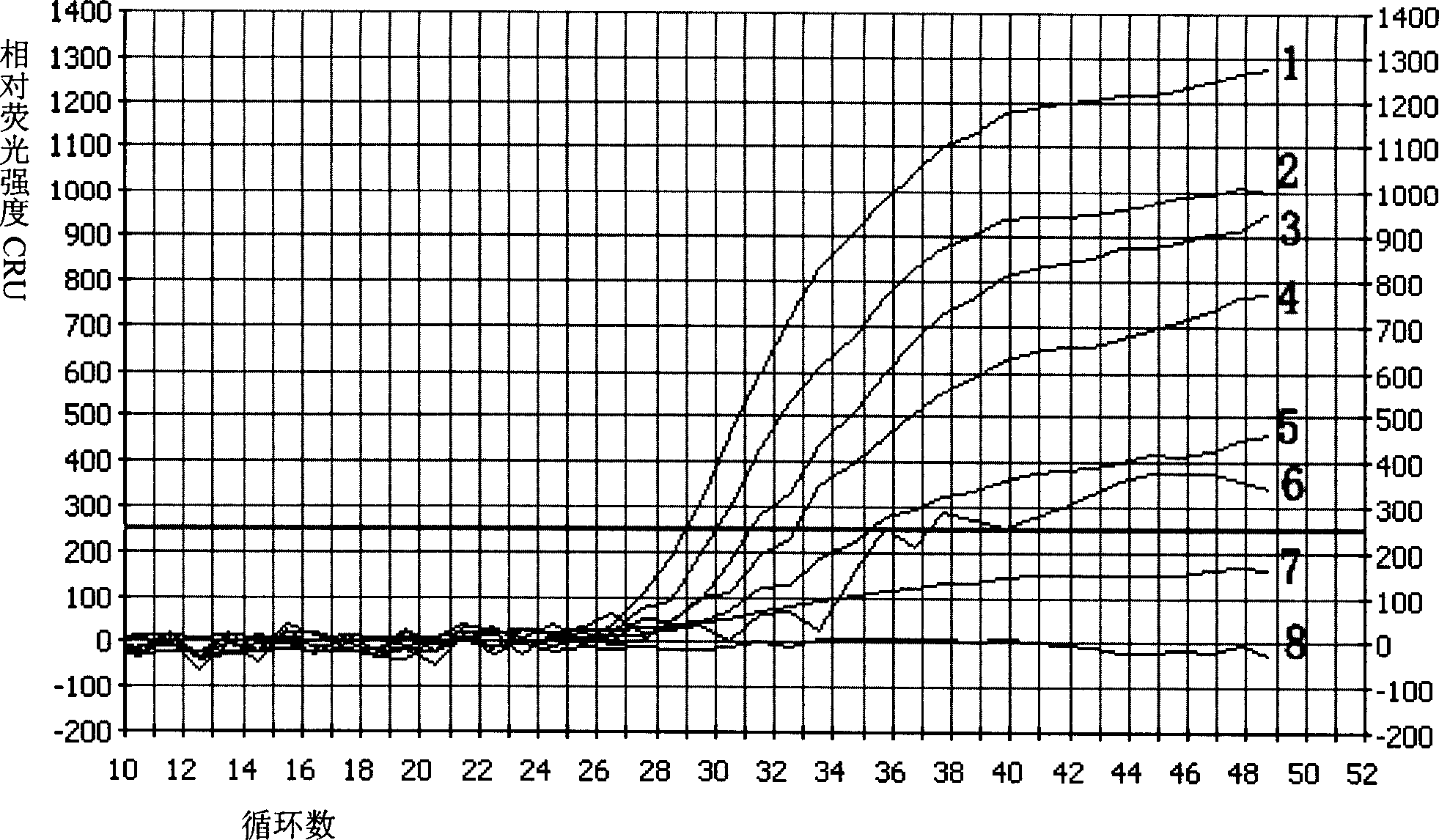
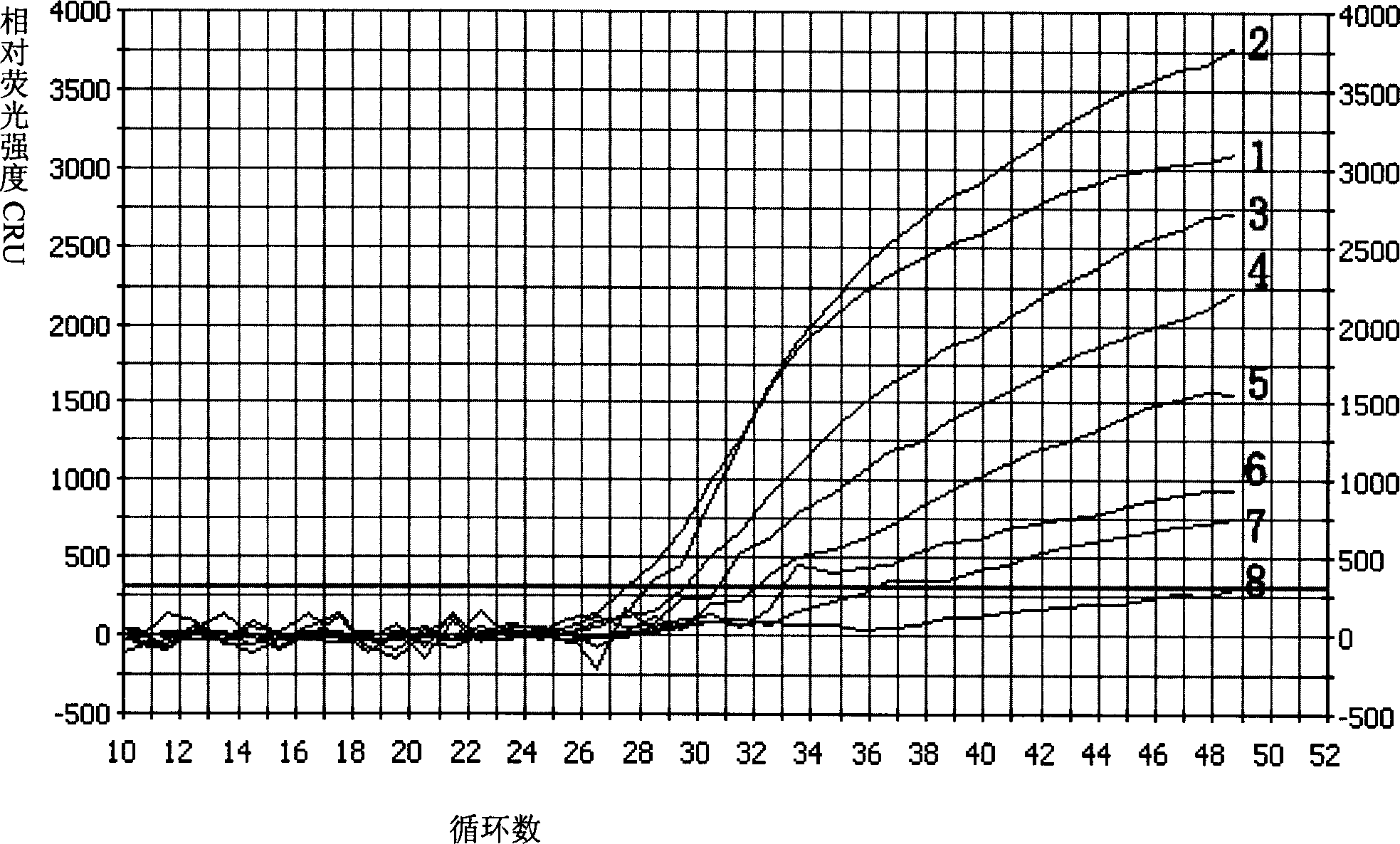
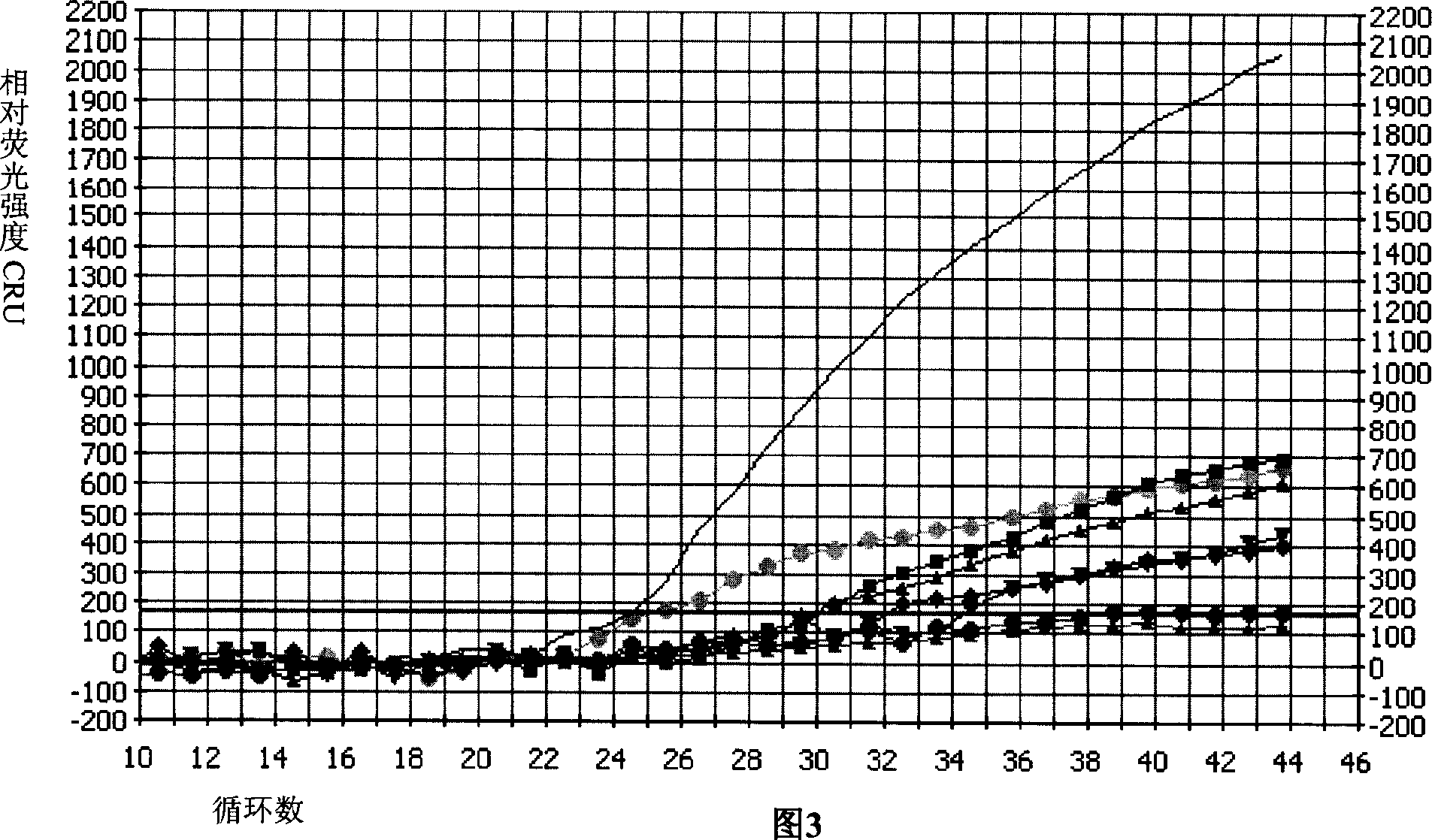
Multiple fluorescence PCR detection reagent for detecting pathogenicity of streptococcus suis serotype 2 and method
A technology for Streptococcus suis and detection reagents, applied in biochemical equipment and methods, microbe determination/inspection, etc., can solve problems such as cumbersome methods and difficulty in obtaining reference serum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Design and synthesis of embodiment 1 primers and probes
[0052] According to the published sequence of two virulence factors MRP (GenBank No.X64450) and EF (GenBank No.A24023) coding genes of Streptococcus suis type 2, primer pairs and probes were designed using the probe design software Beacon Designer 2.1. The primer sequences are:
[0053] MRP (Forward): 5'-AGACCGTCCAAAAGTACCTTACC
[0054] MRP (reverse): 5'-CATTTGTTCCTGGTGTCGTTGG, the length of the amplified product is 80bp;
[0055] The sequence of the probe is: 5'-TGACCCAACAGAGCCAGACGAGCC
[0056] The 5' end of the probe was labeled with the reporter fluorescent dye JOE, and the 3' end was labeled with the quencher fluorescent dye Eclipse.
[0057]EF (Forward): 5'-ACAGTGCAGAAGCAGTAGGC
[0058] EF (reverse): 5'-GAACATCTTGGGCGATTGAACC, the length of the amplified product is 139bp;
[0059] The sequence of the probe is: 5'-ACGCCTTTGTCCTCTGCCGATTGC
[0060] The 5' end of the probe was labeled with the reporter fl...
Embodiment 2
[0062] The extraction of embodiment 2DNA
[0063] 2.1 Bacteria isolation and cultivation
[0064] In the P3 laboratory of the Academy of Military Medical Sciences of the Chinese People's Liberation Army, the isolation and culture procedures of Streptococcus suis recommended by the CDC (Chinese Center for Disease Control and Prevention) were carried out. Tonsil swabs were used to collect the disease materials of the pigs to be tested, and after being brought back to the laboratory, sheep blood agar plates were inoculated respectively. After culturing at 37°C for 24 hours, pick suspicious colonies and inoculate them in Pickle's enrichment solution, culture at 37°C for 24 hours, then inoculate in serum broth, culture on a shaker at 37°C, perform Gram staining and microscopic examination, and inactivate for later use.
[0065] 2.2 Extraction of tissue genomic DNA
[0066] The tonsils, muscles and other tissues of the pigs to be tested and healthy pigs were aseptically collected....
Embodiment 3
[0076] The establishment of embodiment 3 real-time (Real-time) PCR amplification method
[0077] 3.1 PCR reaction system
[0078] The primer pair and probe were dissolved in sterilized double distilled water, the concentration of MRP / EF upstream and downstream primers was 20 mM, and the concentration of differently labeled MRP / EF probes was 10 mM. Then prepare the reaction master mix according to the following ratio:
[0079] 20mM MRP / EF upstream and downstream primers 0.5μl each, probe 0.5μl each, 10×PCR buffer (Mg 2+ Free) 2.5μl, 25mM MgCl 2 3.0μl, 2.5mM dNTP 2μl, stored at 4°C for later use.
[0080] Before use, take 10.5 μl of the above premix, add 0.5 μl of 5 U / μl Taq DNA polymerase, then add 1 μl of genomic DNA of the tissue to be tested as a template, and add sterilized double distilled water to make the volume to 25 μl.
[0081] 3.2 PCR reaction conditions
[0082] After putting the sample tube into the iCycler IQ Real-time PCR instrument of Bio-Rad Company, set ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com