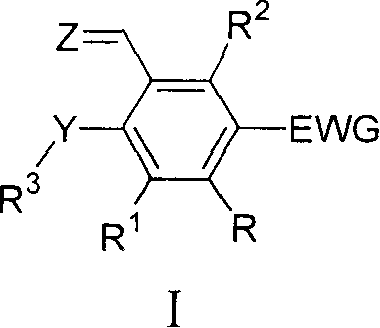
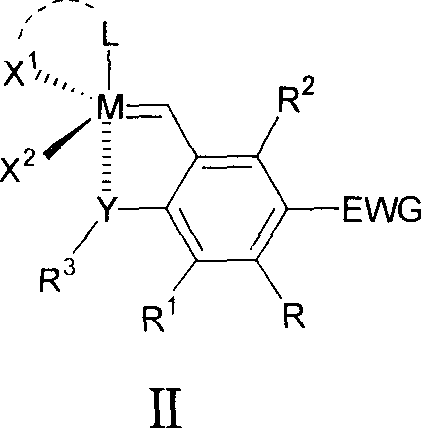
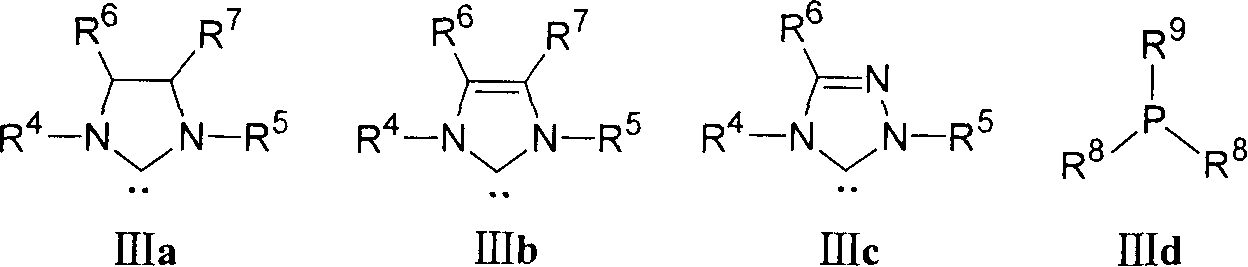
Ruthenium complex compound ligand, ruthenium complex compound, solid carrying ruthenium complex catalyst and preparation method and use thereof
A ruthenium complex and ligand technology, applied in the field of ruthenium complex catalysts, can solve the problems of insufficient catalytic activity and stability, difficult separation of catalysts, and easy decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Example 1 Synthesis of 1-chloro-2-isopropoxy-3-vinyl-benzene and its ruthenium complexes 5a and 5b
[0113] Under argon protection, add tin tetrachloride (36ml 25mL, 0.2mol), 1,2-dichloroethane (240ml 200mL) to a 1 liter three-necked flask equipped with a dropping funnel, mechanical stirring and a thermometer, and cooled to -50°C with a dry ice acetone bath. Start to add tributylamine (72ml 50mL, 0.2mol) dropwise, keep the temperature of the reaction solution not exceeding -50°C during the dropwise addition, and continue stirring for 1 hour after the dropwise addition is completed. Subsequently, acetylene gas was passed into the reaction solution at -50°C (6 hours), the raw material o-chlorophenol (6.50 g, 50 mmol) was added at room temperature, and then heated to 70° C. for 2 hours to obtain a phenol ortho-vinylated product.
[0114] After the reaction, potassium carbonate (25 g) and methanol (100 mL) were added, heated to 60° C. for 1 hour, and then 2N HCl was added ...
Embodiment 2
[0119] Synthesis of embodiment 2 ruthenium complex 5b
[0120] Under argon protection, the more stable ruthenium complex 1b (260mg, 0.30mmol) was weighed instead of 1a and CuCl (75mg, 0.75mmol) into a round bottom flask, and then 5.0mL of dichloromethane was added. Then the ruthenium complex ligand 4a (60 mg, 0.30 mmol) was dissolved in 1.0 mL of dichloromethane, and added to the reaction system. The reaction mixture was stirred at room temperature (20° C.) for 30 minutes, and the reaction was completed. The result was unexpected. There was no raw material 1b in the reaction solution, but the corresponding complex product 5b was not obtained after the complexation reaction, and no molecular ion peak in the reaction solution was observed by mass spectrometer (MS). Thin plate chromatography (TLC) also did not reveal the green product 5a.
Embodiment 3
[0121] Example 3 Synthesis of ruthenium complexes 5e-5i
[0122] Using fluorine (4e), fatty group (4g), nitro group (4h), aminosulfonyl group (4i) etc. to replace the substitution position of the isopropoxy ortho position of chlorine, the corresponding complex 5e-5i is also not obtained, The mass spectrometer did not observe the molecular ion peak in the reaction solution, and the green product 5e-5i was not found by thin-plate chromatography (TLC). Its conclusion can be concluded that if the ortho position of isopropoxy group has electron-withdrawing functional group halogen, aliphatic group, nitro group, aminosulfonyl group, etc., the substituted styrene cannot form a stable ruthenium complex. It can be seen that although Hoveyda et al. first prepared ruthenium complexes (10a and 10b) generated by isopropoxystyrene ligands, they did not study in depth the significant impact of different substituents on the stability of such complexes .
[0123] The test results prove that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com