Recombinant protein Rv3425 and its application of in preparing tuberculosis kit
A rv3425, recombinant protein technology, applied in the field of genetic engineering and diagnostic reagents, can solve the problems of patients with immune system damage, low immunity, and weakened diagnostic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: Construction of recombinant plasmid pET32a-Rv3425
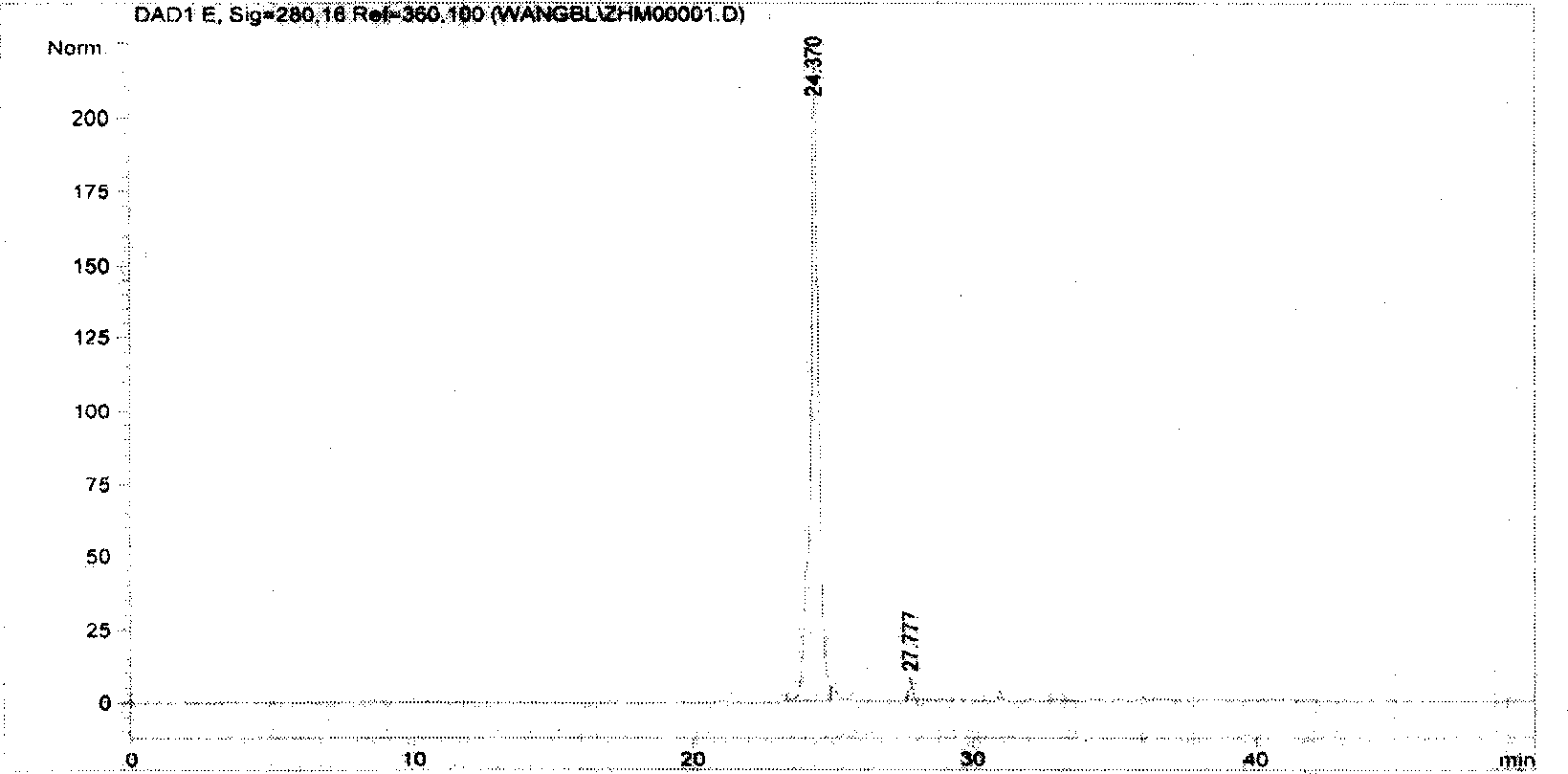
[0057] Primer Rv3425(P 上 : 5'ACGGGATCCATGCATCCAATGATACCA;P 下 : 5' ATAAGCTTCTACCCGCCCCTGTAGATCTG). Using the H37Rv strain genomic DNA as a template, the Rv3425 gene was amplified by PCR ( figure 1 ). The PCR reaction conditions were as follows: pre-denaturation at 98°C for 5 minutes; denaturation at 98°C for 45 seconds, renaturation at 60°C for 60 seconds, extension at 72°C for 1 minute, 30 cycles; extension at 72°C for 10 minutes. The PCR reaction product of the Rv3425 gene was purified and digested with BamH I and Hind IH, and cloned into the restriction site of BamH I and Hind III of pET32a plasmid. Sequencing showed that the inserted sequence was completely consistent with the corresponding gene sequence of the whole genome of H37Rv tuberculosis in Gene Bank (Rv3425 gene accession number BX842583.1; protein accession number: CAE55596.1).
Embodiment 2
[0058] Embodiment 2: Expression and purification of Rv3425 protein
[0059] Preparation and transformation of Escherichia coli BL21 competent cells. The method is as follows: Inoculate 1% of E. coli into 50ml LB culture medium, 200rpm, overnight at 37°C; transfer to a 50ml centrifuge tube, 0°C, 10min, centrifuge at 6000rpm, 4°C for 10min, discard the supernatant; collect the bacteria, gently Vibrate the bacteria block; slowly add 10mL pre-cooled CaCl 2 (100mmol / L) to suspend the bacteria, ice-bath for 20min, and discard the supernatant as above. Slowly add 100mmol / L pre-cooled CaCl 2 300μL was mixed gently and put into a 1.5ml Eppendorf tube, and kept on ice for 2h. Store at 4°C. Add 1 μl of the identified recombinant plasmid to 20 μL of Escherichia coli competent cells and keep it on ice for 45 minutes. Shake 3 times in the middle to prevent the recipient bacteria from sinking to the bottom. 37°C water bath for 5 minutes. Quickly ice-bath for 2 minutes, then add 800 μl...
Embodiment 3
[0060] Example 3: Detection of IgG reaction of recombinant protein Rv3425 to serum of healthy controls, pulmonary tuberculosis and extrapulmonary tuberculosis patients.
[0061] Use ELISA indirect method to detect the IgG reaction against Rv3425 in different serum samples. The specific steps are: 96-well plate is coated with antigen Rv3425 (concentration: 5 μg / mL) overnight, and then the plate is washed 3 times with 400 μL / well of PBST , each time for 3 minutes, followed by blocking with 1% BSA in PBS (350 μL / well) and incubating at 37°C for 2 hours, then washing the plate three times with PBST 400 μL / well, each time for 3 minutes, and then adding 1:100 diluted to-be-tested Serum (100 μL / well) was incubated at 37°C for 1 h, and the plate was also washed 5 times with 400 μL / well of PBST for 5 minutes each time; then 400ul of freshly diluted enzyme-labeled antibody (1:1000 dilution) was added. Incubate at 37°C for 1 hour, and then wash the plate 5 times with 400 μL / well of PBST,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com