Quality control objects of whole blood, and production method
A production process and blood quality technology, applied in the field of whole blood quality control substances and production processes, can solve problems such as unfavorable cell membrane protection, good indoor quality control results, and uncontrolled instrument status, and achieve a reasonable concentration control level and bottle-to-bottle difference. Small, the effect of controlling the difference between bottles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
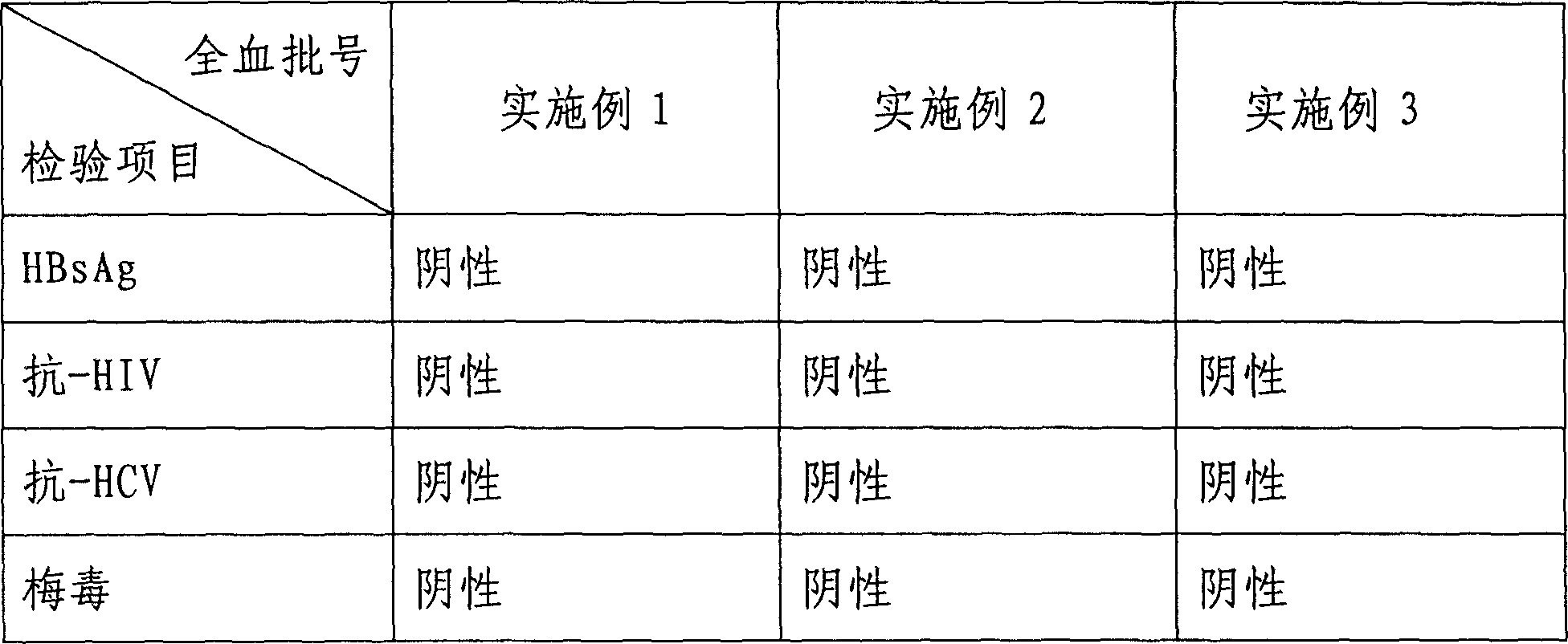
Examples
Embodiment 1
[0015] Whole blood quality control substance formula of the present invention:
[0016] 1) Type O blood 400ml
[0017] 2) Anti-aggregation agent 35ml / 400ml blood
[0018] Wherein, antipolymerization agent formula is by weight of each component in 1 liter of water:
[0019] Citric acid 2.22g / L
[0020] Sodium citrate 26.3g / L
[0021] EDTA-Na 60g / L
[0022] Glucose 25g / L
[0023] Sodium dihydrogen phosphate 2.25g / L
[0024] Adenine 0.275g / L
[0025] 3) Fixative 0.8ml / 400ml blood
[0026] Wherein, fixative formulation is by volume ratio:
[0027] Ethanol: glutaraldehyde = 30ml: 1.8ml
[0028] 4) Protective agent 15ml / 400ml blood
[0029] Wherein, the protective agent formula is by weight of each component contained in 1 liter of water:
[0030] Aspirin 1.7g
[0031] Dipyridamole 0.3g
[0032] Penicillin - 13 million units
[0033] Firstly prepare the anti-aggregation agent, fixative and protective agent according to the above proportions, mix the three components ev...
Embodiment 2
[0035] Whole blood quality control substance formula of the present invention:
[0036] 1) Type A blood 400ml
[0037] 2) Anti-aggregation agent 50ml / 400ml blood
[0038] Wherein, antipolymerization agent formula is by weight of each component in 1 liter of water:
[0039] Citric acid 3.0g / L
[0040] Sodium citrate 35.0g / L
[0041] EDTA-Na 90g / L
[0042] Glucose 35.0g / L
[0043] Sodium dihydrogen phosphate 1.25g / L
[0044] Adenine 0.175g / L
[0045] 3) Fixative 1.5ml / 400ml blood
[0046] Wherein, fixative formulation is by volume ratio:
[0047] Ethanol: glutaraldehyde = 60ml: 2.2ml
[0048] 4) Protective agent 8ml / 400ml blood
[0049] Wherein, the protective agent formula is by weight of each component contained in 1 liter of water:
[0050] Aspirin 1.0g
[0051] Dipyridamole 0.6g
[0052] Penicillin - 26 million units
[0053] Firstly prepare the anti-aggregation agent, fixative and protective agent according to the above proportions, mix the three components ev...
Embodiment 3
[0055] Whole blood quality control substance formula of the present invention:
[0056] 1) Type B blood 400ml
[0057] 2) Anti-aggregation agent 80ml / 400ml blood
[0058] Wherein, antipolymerization agent formula is by weight of each component in 1 liter of water:
[0059] Citric acid 4.0g / L
[0060] Sodium citrate 32.0g / L
[0061] EDTA-Na 110g / L
[0062] Glucose 45.0g / L
[0063] Sodium dihydrogen phosphate 4.00g / L
[0064] Adenine 0.375g / L
[0065] 3) Fixative 10ml / 400ml blood
[0066] Wherein, fixative formulation is by volume ratio:
[0067] Ethanol: glutaraldehyde = 90ml: 3.0ml
[0068] 4) Protective agent 15ml / 400ml blood
[0069] Wherein, the protective agent formula is by weight of each component contained in 1 liter of water:
[0070] Aspirin 2.5g
[0071] Dipyridamole 0.8g
[0072] Penicillin - 20 million units
[0073] First, prepare anti-polymerization agent, fixative and protective agent respectively according to the above proportions, mix the three com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com