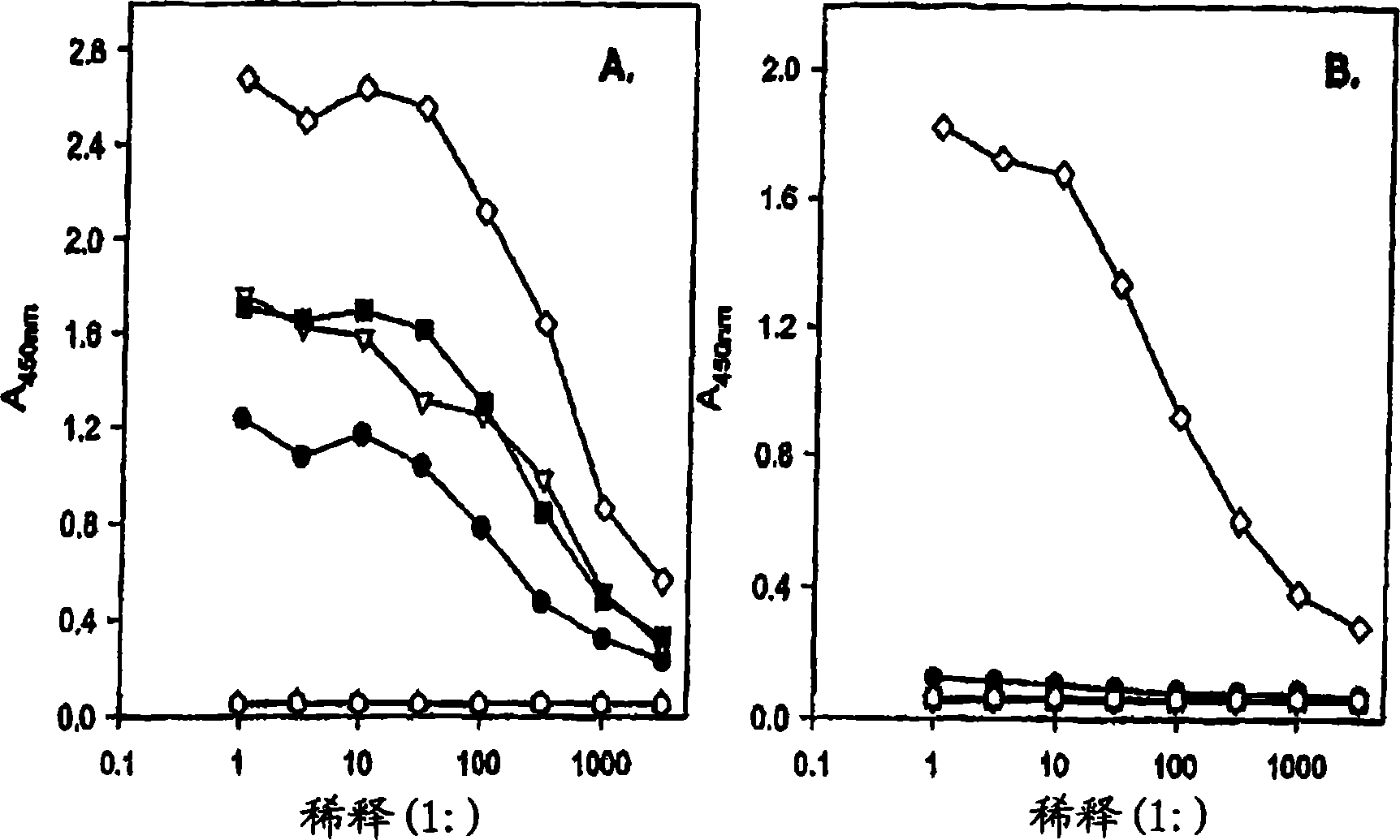
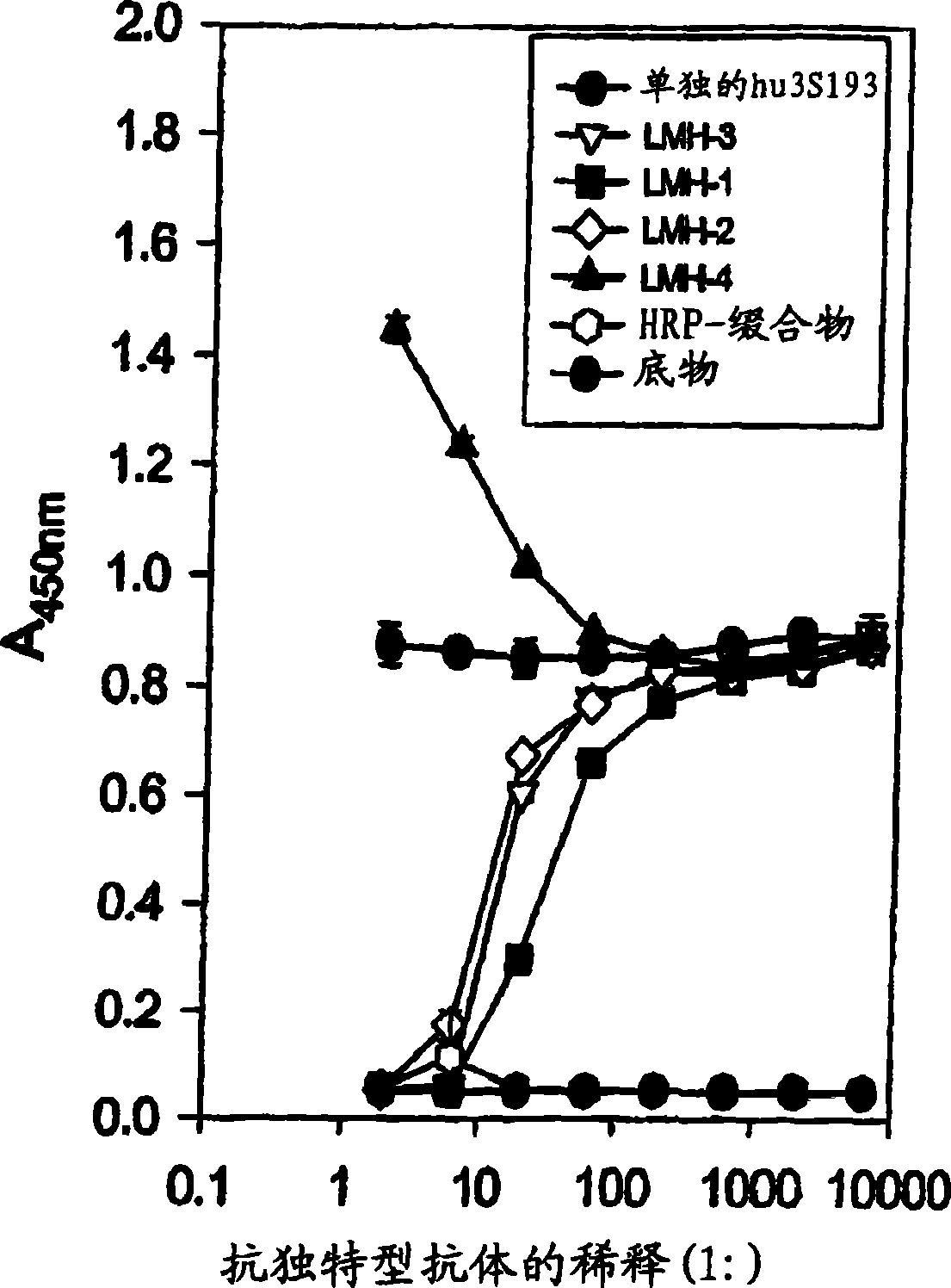
Anti-Lewis Y anti-idiotypic antibodies and uses thereof
An anti-idiotypic antibody and anti-idiotype technology, applied in the field of molecular biology, can solve the problem of undetectable HAHA immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] cell culture
[0038] All analytical grade reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA), unless otherwise noted.
[0039] The SP2 / 0-Ag14 plasmacytoma cell line was purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA). SP2 / 0 and selected hybridoma clones were cultured in standard RPMI-1640 medium; it was a medium containing 10% heat-inactivated fetal calf serum (FCS, TRACE Biosciences Pty Ltd, Sydney, Australia), 100 μg / ml streptomycin , 100 U / ml penicillin (Gibco) and 4 mM L-glutamine in RPMI-1640 medium (Gibco Invitrogen Corp., Mt Waverley, Victoria, Australia). The initial few passages of fusion products were cultured with RPMI-1640 containing 20% FCS, penicillin, streptomycin and L-glutamine as described above. Hybridomas were selected using HAT medium; it was medium supplement containing 20% FCS, HAT (hypoxanthine, aminopterin, thymine), OPI (oxaloacetate, pyruvate, insulin) (Sigma ), penicillin, streptomycin, L...
Embodiment 2
[0041] Antibodies for Immunization and Screening
[0042]Humanized anti-Lewis Y IgG1 antibody hu3S193 (lot: D01097, 10 mg / ml PBS), murine 3S193 (mu3S193), control isotype-matched humanized mAb huA33 (lot: 5GMA33 10 mg / ml) and an irrelevant isotype-matched The mouse:human chimeric IgG1 antibody was provided by Biological Production Facility, Ludwig Institute for Cancer Research, Melbourne, Australia. Purified human IgG was purchased from Sigma Chemical Co. F(ab)' was prepared by digestion with immobilized pepsin according to the manufacturer's instructions (Pierce, Rockford, IL, USA). 2 Fragments, undigested hu3S193 and Fc-containing fragments were purified by passing through a 1 ml protein-A column (Pharmacia Biotech, Uppsala, Sweden). Purified fragments were concentrated to 1 mg / ml in Millipore centrifugal filters (Biomax 10k membrane, Millipore, Sydney, Australia).
Embodiment 3
[0044] Immunization program
[0045] Six to eight week old female BALB / c mice purchased from the Breeding Facility, Walter and Eliza Hall Institute, Melbourne, Australia were fed ad libitum food and water. All procedures performed on animals used in this study were approved by the Animal Ethics Committee of Austin and Repatriation Medical Centre. Pre-immune venous blood samples (200 μl) were collected from each animal and clotted for 1 hour at room temperature and overnight at 4°C. The resulting serum (about 100 μl / mouse) was collected and stored at -20°C.
[0046] The immunization step was performed with six mice. On day 0, BALB / c mice were immunized by intraperitoneal injection of 200 μl containing 40 μg of purified hu3S193:complete Freund's adjuvant (1:1, v:v). Mice received booster injections on days 14 and 28 in incomplete Freund's adjuvant containing 40 μg hu3S193. On day 35, post-immunization blood samples were collected from the tail vein, and serum titers of mouse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com