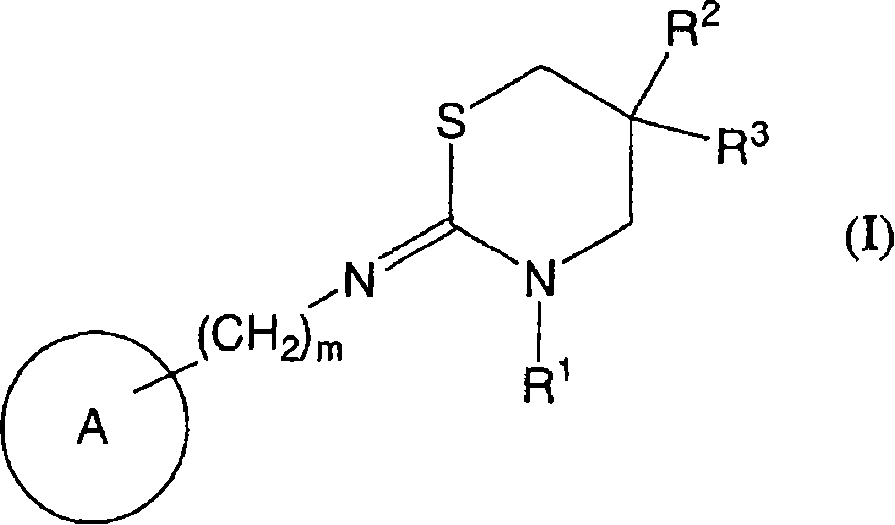
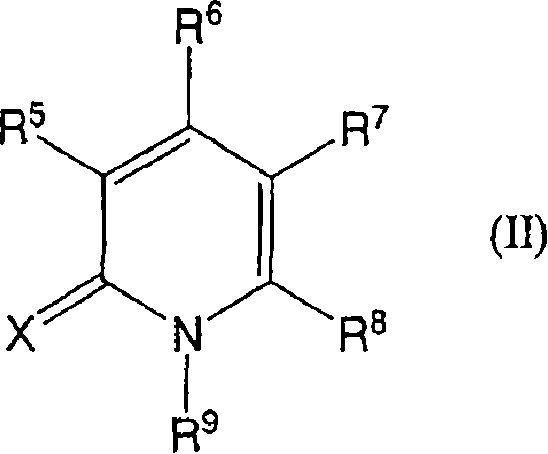
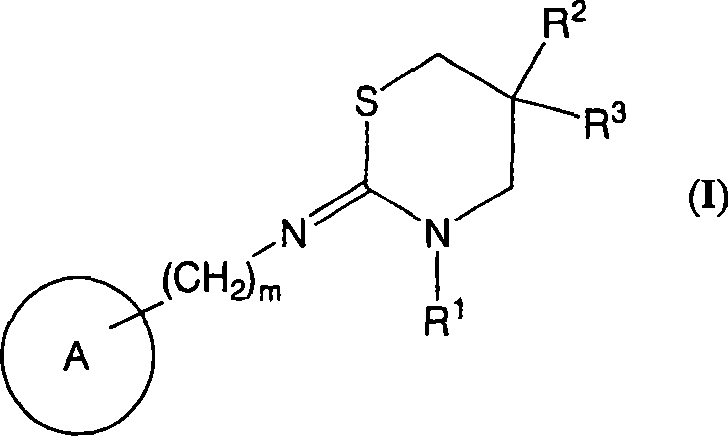
Novel use of cannabinoid receptor agonist
An inhibitory and irritative technology, applied in the direction of anti-inflammatory agents, medical preparations containing active ingredients, non-central analgesics, etc., can solve the problem of no description of inhibitory active mucus secretion inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment Embodiment 1、2 and 3
[0277] Experimental Examples 1, 2 and 3: Effects on antigen-induced bronchial hyper-irritability, inflammatory cell infiltration and mucus secretion (acute model) in BN rats
[0278] Antigen-induced bronchial hyper-irritability in BN rats: Brown Norway (BN) rats (Charles River) were injected intraperitoneally with 1 mL of a mixture containing aluminum hydroxide gel (1 mg) and egg white protein (0.1 mg, OVA). Japan) was actively sensitized. Ten days later, the antigen challenge was performed by inhaling the atomized 1% OVA solution using an ultrasonic nebulizer for 30 minutes. 24h after antigen challenge, under pentobarbital sodium anesthesia (80mg / kg, intraperitoneal injection), rats were injected intravenously with ACh by increasing the dose of ACh every 5min, and then by Konzett&Rssler with some modifications The method measures the bronchoconstrictor response observed immediately after each ACh injection. Briefly, the rat’s trachea was cut and a cannula was connected to the lu...
experiment Embodiment 4、5 and 6
[0286] Experimental Examples 4, 5, and 6: Effects on antigen-induced bronchial hyper-irritability, inflammatory cell infiltration and mucus secretion (chronic model) in BN rats
[0287] Antigen-induced bronchial hyper-irritability in BN rats: BN rats are actively sensitized by intraperitoneal injection of a mixture containing aluminum hydroxide gel and egg white protein. Twelve days later, the antigen challenge was performed by using an ultrasonic nebulizer (NE-U12, Omron) to inhale a nebulized 1% OVA solution or physiological saline for 30 minutes. In order to establish a model of chronic bronchial hyperstress, the procedure was repeated 3 times at 1 week intervals. From the day of the third antigen challenge, the test compound was orally administered for 8 days. On the day of the third antigen challenge, 1 h before the challenge, the test compound was administered. One hour after the last administration of the test compound, a fourth antigen challenge was performed. 24 hours aft...
experiment Embodiment 7、8 and 9
[0294] Experimental Examples 7, 8 and 9: Effects on antigen-induced bronchial hyper-irritability, inflammatory cell infiltration and mucus secretion (acute model) in guinea pigs
[0295]Excessive bronchial irritability induced by antigen in guinea pigs: At 1 week intervals, the guinea pigs trapped in the aeration chamber ( Charles River Japan) was actively sensitized. One week later, antigen challenge was performed by inhaling atomized 1% OVA generated by an ultrasonic nebulizer for 5 minutes. One hour before antigen challenge, the test compound was administered orally. In addition, guinea pigs were treated with diphenhydramine (10 mg / kg, intraperitoneal injection) and antihistamines 10 min before antigen challenge to prevent allergic death of the animals. 24h after antigen challenge, under urethane anesthesia (1.4g / kg, peritoneal injection), guinea pigs were injected intravenously with ACh by increasing the dose of ACh every 5min, and then by the Konzett & Rssler method with som...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com