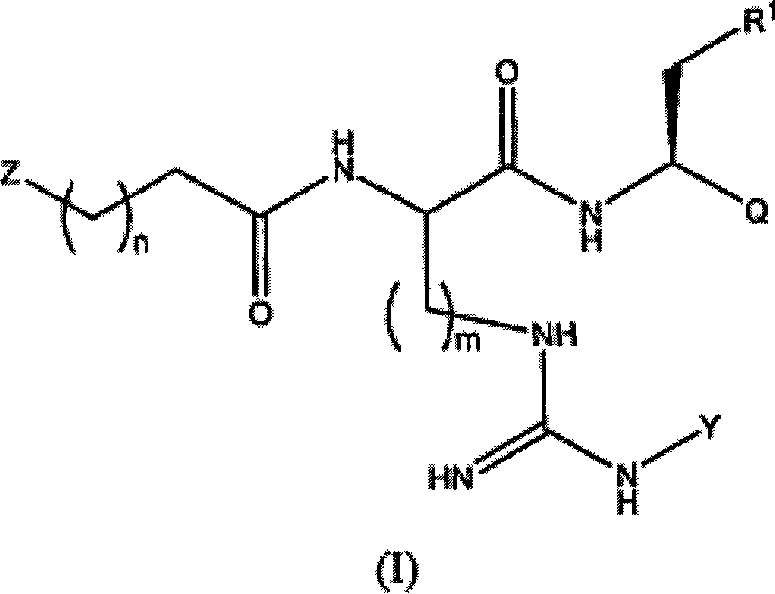
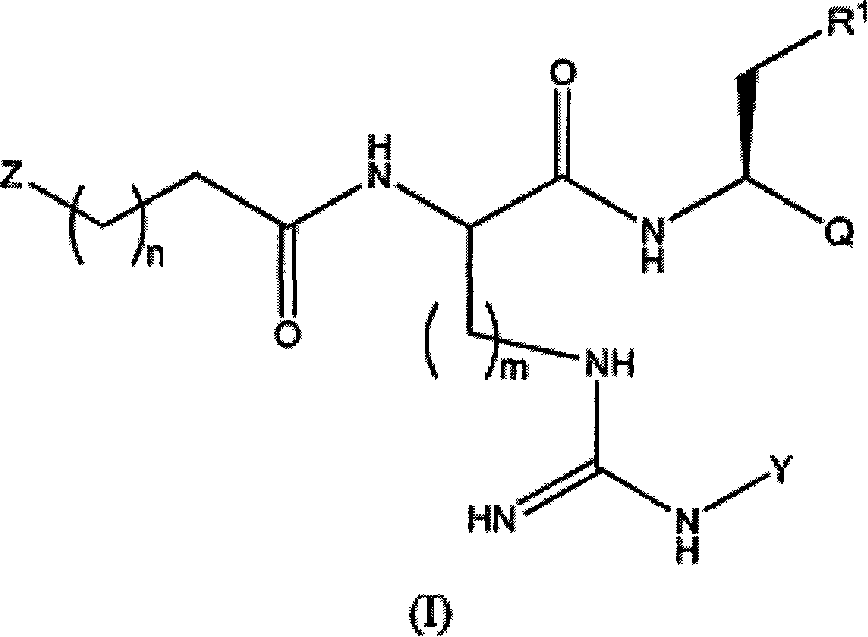
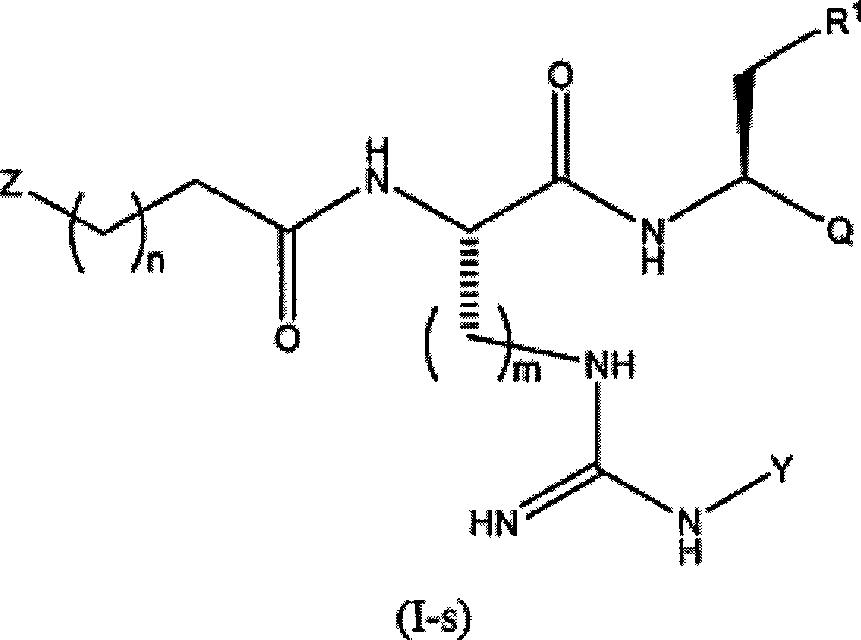
Proteasome inhibitors and methods of using the same
A technology of stereoisomerism and compounds, applied in chemical instruments and methods, extracellular fluid diseases, compounds containing elements of group 3/13 of the periodic table, etc., can solve problems such as reducing the degradation rate of p53 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] (1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methylene-1,3,2-benzodioxanborol- Synthesis of 2-yl]-3-methylbutylamine hydrochloride
[0163] Step 1: 2-(2-Methylpropyl)-(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methylene-1,3,2 - Benzodioxin borole
[0164]
[0165] A mixture of (+)-pinanediol (23.9 g, 0.140 mol) and 2-methylpropylboronic acid (15 g, 0.147 mol) in diethyl ether (300 mL) was stirred at room temperature for 24 hours. The mixture was dried over anhydrous sodium sulfate and purified by column chromatography (silica gel 230-400 mesh), eluting with a mixture of hexane:ethyl acetate 90:10. The product was obtained as a clear oil (32.6 g, 94% yield).
[0166] 1 H NMR (DMSO-d 6 ): 4.28 (1H, dd, J = 8.8Hz, 2.0); 2.30 (1H, m); 2.18 (1H, m); 1.96 (1H, t, J = 5.3); 1.86 (1H, m); 1.78 ( 1H, set, J = 6.8); 1.68 (1H, m); 1.30 (3H, s); 1.25 (3H, s); 1.01 (1H, d); 0.9 (6H, d, J = 6.6); 3H, s); 0.69(2H, m).
[0167] Step 2: 2-[(1S)-1-Chloro-3-methyl...
Embodiment 2
[0180] N-[(1S)-1-[[[(1R)-1-[(3aS,4S,6S,7aR)hexahydro-3a,5,5-trimethyl-4,6-methylene-1 , 3,2-Benzodioxanborol-2-yl]-3-methylbutyl]amino]carbonyl]-4-[[imino(nitroamino)methyl]amino]butyl-carbamic acid 1 , 1-Dimethylethyl Ester
[0181]
[0182] Method A: HOAt / HATU
[0183] To BocNH(NO 2 ) ArgOH (15.7g, 49.3mmol) in anhydrous DMF (100ml) solution, add HATU (O-(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium Hexafluorophosphate; 18.7 g, 49.3 mmol) and HOAt (1-hydroxy-7-azabenzotriazole; 6.71 g, 49.3 mmol). The mixture was cooled to 0°C and N-methylmorpholine (13.6ml, 0.123mol) was added. After 10 minutes, (1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5trimethyl-4,6-methylene-1,3, 2-Benzodioxaborol-2-yl]-3-methylbutylamine hydrochloride (12.4 g, 41.1 mmol). The cooling bath was removed and the mixture was stirred at room temperature for 4.5 hours. The mixture was diluted with ethyl acetate (800ml), washed with 2% citric acid solution (2×150ml), 2% NaHCO 3 solution (2 x 150ml...
Embodiment 3
[0188] N-[(1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a,5,5-trimethyl-4,6-methylene-1,3,2-benzobis Oxaborol-2-yl]-3-methylbutyl]-(2S)-2-amino-5-[[imino(nitroamino)methyl]amino]pentanamide hydrochloride
[0189]
[0190] Method A
[0191] A solution of 4N hydrochloric acid in dioxane (15ml) was added to N-[(1S)-1-[[[(1R)-1-[(3aS,4S,6S,7aR)-hexahydro-3a ,5,5-trimethyl-4,6-methylene-1,3,2-benzodioxanborol-2-yl]-3-methylbutyl]amino]carbonyl]-4-[[ 1,1-Dimethylethyl imino(nitroamino)methyl]amino]butyl]-carbamate (4.04g, 7.06mmol) in dioxane (40ml) and diethyl ether (7ml) solution of the mixture while maintaining cooling at 0 °C. The reaction mixture was warmed to room temperature and stirring was continued for 4 hours. The solvent was removed by rotary evaporation, the residue was treated with diethyl ether (50ml) and the mixture was stirred at room temperature for 3 days. The resulting solid was collected by filtration to yield 3.18 g of pure product (90% yield).
[0192] Method B ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com