Abrotine, its derivative dihydro-abrotine, artemether, arteether and arte sunate in use of pharmacy
A technology of dihydroartemisinin and artemether, which is applied in the field of pharmacy to achieve the effects of widening the scope of adaptation, good medicinal prospects, and significant in vivo and in vitro anti-inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0022] Experimental example 1. The dose-effect relationship of artemisinin, dihydroartemisinin, artemether and artesunate in inhibiting the release of cytokines induced by CpG-ODN.
[0023] Culture mouse macrophage RAW264.7, adjust the concentration of cell suspension to 2×10 6 / ml, added to a 48-well plate, 0.4ml per well. Set at 37°C CO 2 After culturing in the incubator for 4 hours, the cells were allowed to adhere to the wall, and different concentrations of artemisinin (5, 10, 20, 40, 80 μg / ml) or dihydroartemisinin, artemether, and artesunate were added. Add 10 μg / ml stimulatory CpG ODN (5′-TCC ATG ACG TTC CTG ACG TT-3′), culture in 37°C, CO2 incubator for 4 hours, take cell culture supernatant to test cytokine TNF-α, and then After 4 hours, the cell culture supernatant was taken to test the cytokine IL-6. The concentration of TNF-α and IL-6 in the cell culture supernatant was measured by double-antibody sandwich ELISA method, and the effect of artemisinin or its deri...
experiment example 2
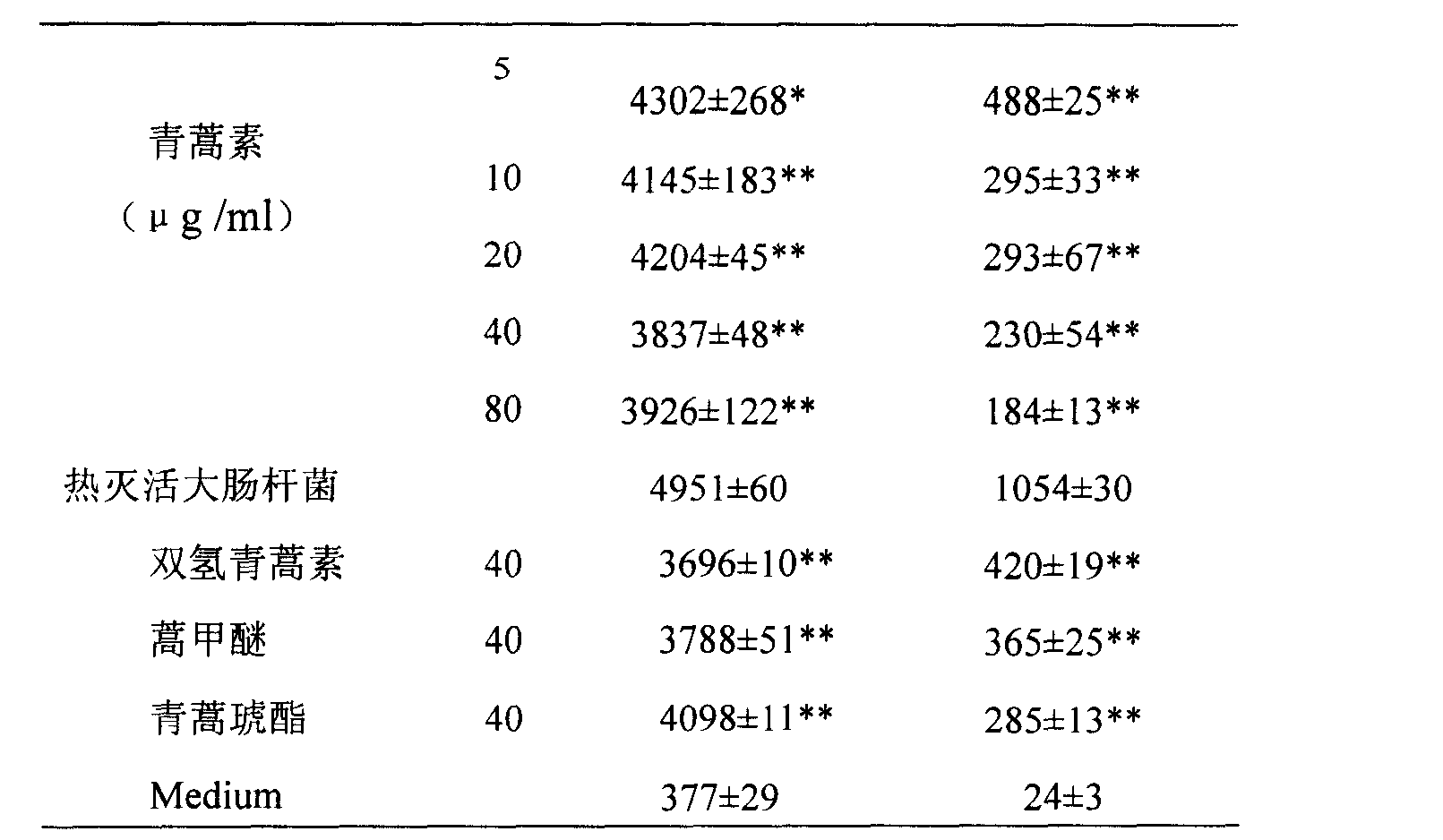
[0027] Experimental example 2. The dose-effect relationship of artemisinin, dihydroartemisinin, artemether and artesunate in inhibiting the release of cytokines induced by heat-killed Escherichia coli.
[0028] Culture mouse macrophage RAW264.7, adjust the concentration of cell suspension to 2×10 6 / ml, added to a 48-well plate, 0.4ml per well. Set at 37°C CO 2 After culturing in the incubator for 4 hours, the cells were allowed to adhere to the wall, and different concentrations of artemisinin (5, 10, 20, 40, 80 μg / ml) or 40 μg / ml of dihydroartemisinin, artemether or artesunate were added, After 2 hours add 1 x 10 6 / ml heat-inactivated Escherichia coli, cultured in a 37°C, CO2 incubator for 4 hours, then took the cell culture supernatant to test the cytokine TNF-α, and after another 4 hours, took the cell culture supernatant to test the cytokine IL-6. The concentration of TNF-α and IL-6 in the cell culture supernatant was measured by double-antibody sandwich ELISA method,...
experiment example 3
[0033] Experimental example 3. Time-effect relationship of artemisinin inhibiting cytokine release induced by CpG ODN.
[0034] Culture mouse macrophage RAW264.7, adjust the concentration of cell suspension to 2×10 6 / ml, added to a 48-well plate, 0.4ml per well. Set at 37°C, CO 2 After culturing in the incubator for 4 hours, the cells were allowed to adhere to the wall, and the time of adding CpG ODN was regarded as the 0 time point, and 20 μg / ml artemisinin was added at -4, -2, -1, 0, 1, and 2 hours respectively, and placed at 37°C, After 4 hours of culture in a CO2 incubator, the cell culture supernatant was taken to measure TNF-α, and after another 4 hours, the cell culture supernatant was taken to test the cytokine IL-6. To clarify the ability of artemisinin to inhibit the release of cytokines from RAW264.7 induced by CpG ODN.
[0035] Table 3 The time-effect relationship of artemisinin inhibiting CpG ODN-induced TNF- release from RAW264.7 (x±SD)
[0036]
[0037...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com