Recombinant monoclonal antibody against EGFR
A monoclonal antibody and receptor technology, applied in the field of recombinant human anti-epidermal growth factor receptor monoclonal antibodies, can solve problems such as limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1. Construction and screening of human antibody library
[0040] According to Marks et al. J. Mol. Biol., 222, 581-597; Hoogenboom and Winte, J. Mol. Biol., 227, 381-388; Haidaris CG et al., J Immunol Methods. 2001 Nov 1; 257 (1- 2): 185-202; Griffiths, A.D. et al. EMBO J., 13, 3245-3260 (1994); Nissim, A. et al. The method described in EMBO J., 13, 692-698 (1994) to construct a human antibody library .
[0041] The starting material is the peripheral blood of 2028 healthy people. According to the method provided in the above literature, mRNA is prepared, and the variable region genes of immunoglobulin heavy chain and light chain are amplified in vitro by PCR method, and cloned into bacteriophage The carrier utilizes the characteristics of antibody molecular fragments presented on the surface of phages, and uses EGFR protein (purchased from Jingmei Biological Co., Ltd.) as an antigen to screen specific antibodies to EGFR protein.
[0042] Add 1 ml of the recov...
Embodiment 2
[0050] Example 2. Cloning of Expression Vectors for Antibody Variable Region Coding Sequences
[0051] The cloned bacterial strain obtained in Example 1 was amplified in 100 ml of LB medium, and the plasmid DNA was purified with a plasmid DNA extraction and purification kit from Promega Company.
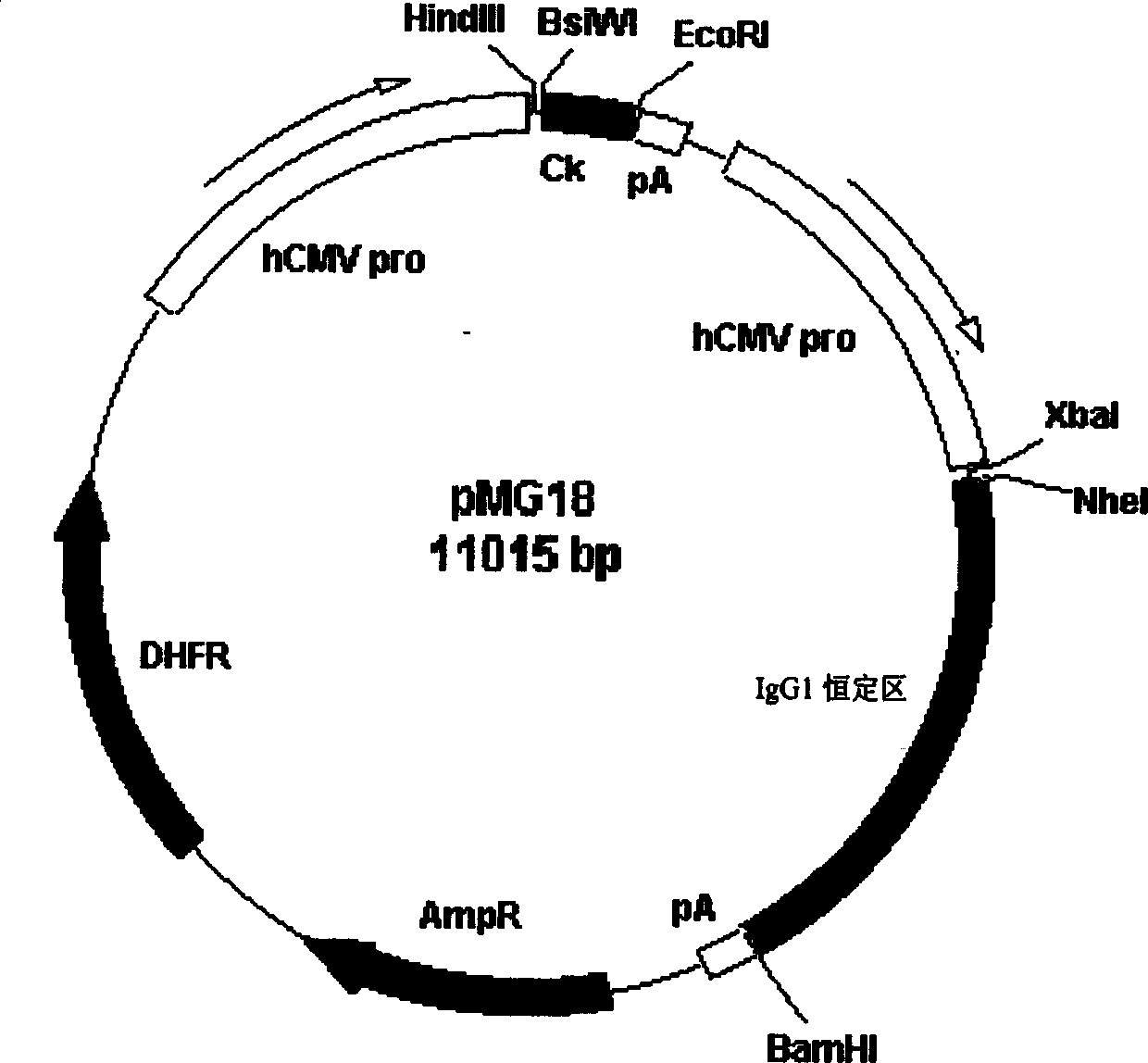
[0052] The above plasmid DNA was digested with XbaI and NheI, and the digested fragments were separated on 1.5% agarose gel electrophoresis, and a band of about 360 bp was taken for gel recovery, and the obtained fragment was the heavy chain variable region coding sequence.
[0053] The above plasmid DNA was digested with HindIII and BsiWI, and the digested fragments were separated on 1.5% agarose gel electrophoresis, and a band of about 320 bp was taken for gel recovery, and the resulting fragment was the light chain variable region coding sequence.
[0054]Then first insert the above heavy chain variable region coding sequence into the expression vector pMG18 (see book DEVELOPMENT ...
Embodiment 3
[0055] Example 3. Transfection of CHO cells and screening of recombinant clones
[0056] The expression vector with the antibody gene constructed in the above-mentioned Example 2 was transferred into the Escherichia coli DH5α strain, and then inoculated in 100 milliliters of LB medium for amplification, and the ultrapure plasmid DNA purification kit (Ultrapure Plasmid DNA Purification Kit) to extract and purify plasmid DNA. The above-mentioned purified plasmid DNA was transfected into CHO cells using the liposome method kit of Invitrogen Company, and the operation was performed according to the manufacturer's instructions.
[0057] The transformed CHO cells were continuously selected on the selection medium for 9 weeks, and finally cultured in extreme dilution on a 96-well plate for 3 consecutive times for monoclonalization.
[0058] The selected monoclonal cell lines were cultured on RPM1641 medium, and the supernatant was subjected to Western blot experiments, and the expre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com