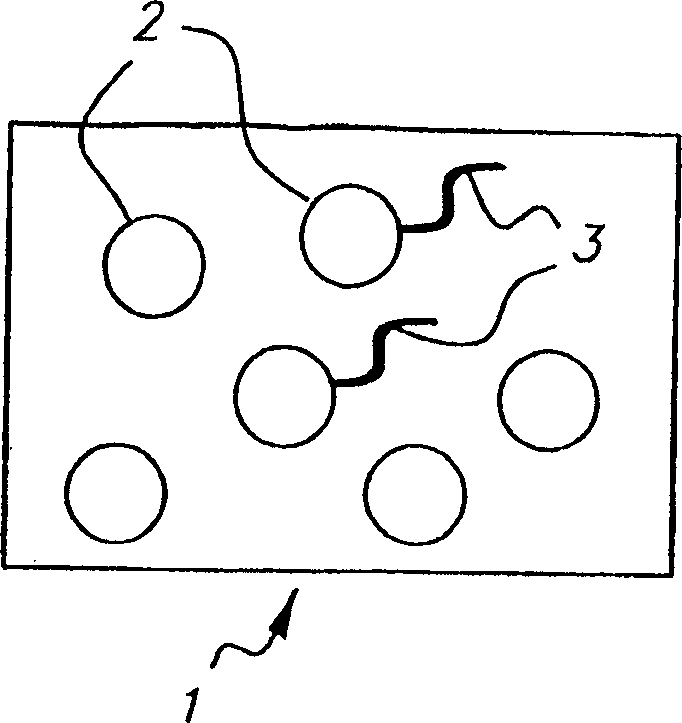
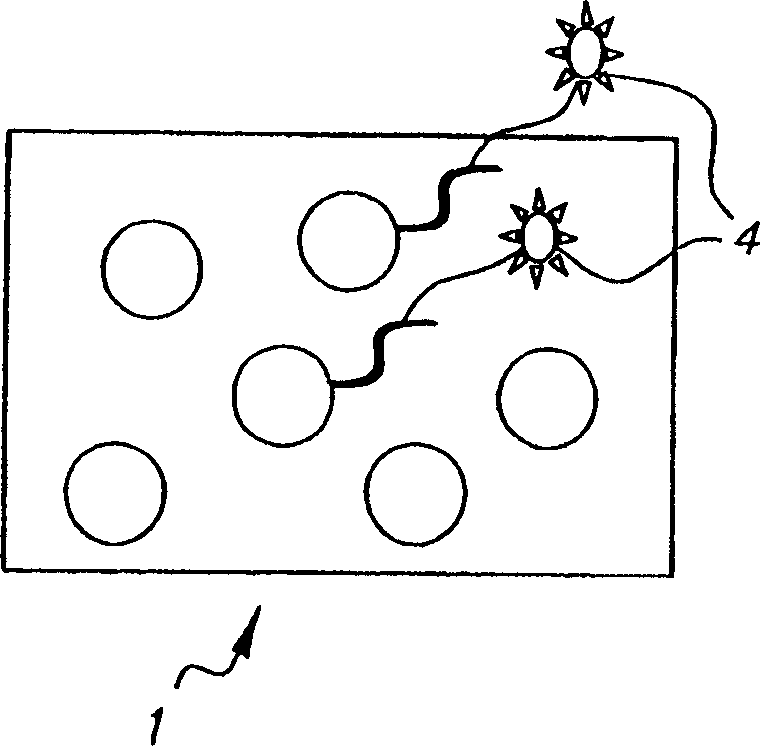
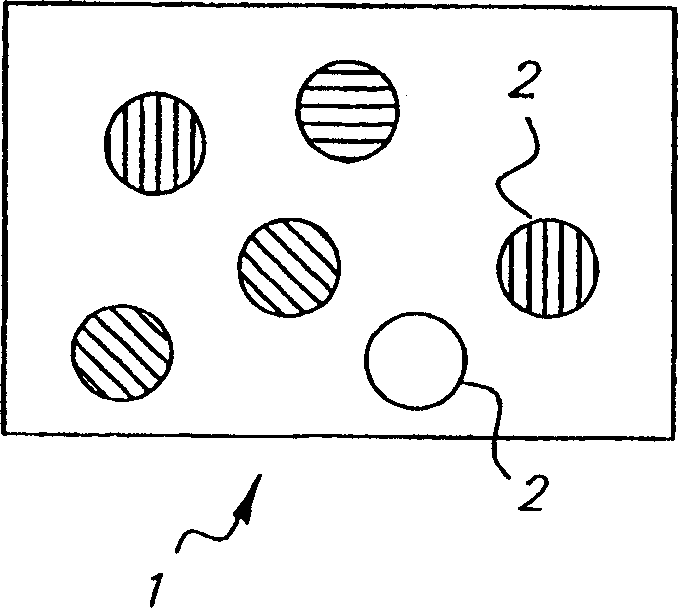
Colorable microspheres for DNA and protein microarray
A microarray and microsphere technology, applied in the field of biological microarrays, can solve the problems of fluorescence signal interference, fluorescence signal intensity suppression, limiting the diversity of color barcode coding of microspheres, etc. The effect of dynamic range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] This example illustrates two methods for loading a photocoupler as a latent colorant into polystyrene microspheres.
[0114] Loading method 1: For the general preparation process, a single coupler, or more than one coupler in a fixed ratio, and different ratios of couplers, coupler solvents, and auxiliary coupler solvents are used to prepare microsphere samples. The cyan coupling agent CYAN1 was loaded by ultrasonic treatment, specifically as follows: 0.08 g of CYAN1 was stirred and dissolved in 0.8 g of cyclohexanone and 0.08 g of tricresyl phosphate (tricresolphosphate). This oil phase was then added to an aqueous phase of 0.48 g FAC-0064 (surfactant) and 6.52 g water with stirring at room temperature. The sample was sonicated for 1 minute to give a milky white dispersion, which was then stirred. An equal amount of 8.0 g of 4% 10 micron polystyrene microspheres was added to the sonicated sample. After mixing, the samples were poured into diafiltration bags and washe...
Embodiment 2
[0119] These examples illustrate the loading of photographic couplers as latent colorants into polystyrene microspheres using in situ polymerization.
[0120]
[0121] Table 1
[0122] Monomer-Coupler 1 Monomer-Coupler 2 Monomer-Coupler 3
[0123] (cyan) (yellow) (magenta)
[0124] beads#
1 (cyan)
2 (yellow)
3 (magenta)
Monomer-Coupler 1 (g)
0.85
-
-
Agent 2 (g)
-
0.85
-
Agent 3 (g)
-
-
0.85
Styrene (ml)
36.6
36.6
36.6
AIBN(g)
0.38
0.38
0.38
Ethanol (ml)
87.5
87.5
87.5
Methyl solution
(ml)
125.0
125.0
125.0
(g)
3.75
3.75
3.75
Average
Grain diameter
(μm)
4.26
4.92
7.54
...
Embodiment 3
[0128] This example demonstrates the attachment of presynthesized single-stranded oligonucleotide probes to the surface of microspheres incorporating coupling agents.
[0129] Wash 100 μl of microspheres (4% w / v) incorporating coupling agent three times in acetate buffer (0.01M, pH 5.0), and mix with 100 μl of 20 mM 2-(4-dimethylcarbamoyl -pyrido)-ethane-1-sulfonate in combination with 10% polyethyleneimine. The mixture was stirred at room temperature for 1 hour and washed three times with sodium borate buffer (0.05M, pH 8.3). Resuspend the beads in sodium borate buffer.
[0130] Dissolve the 5'-amino-C6 modified oligonucleotide DNA probe in 100 μl of sodium borate buffer to a final concentration of 40 nmol. 20 µl of cyanuric chloride in acetonitrile was added to the DNA probe solution, and the total volume was brought up to 250 µl with sodium borate buffer solution. The resulting solution was stirred at room temperature for 1 hour, and then dialyzed against 1 liter of bori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com