Pulse-promoting slow-release capsule and preparing method
A technology for sustained-release capsules and pulse regeneration, which can be used in capsule delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
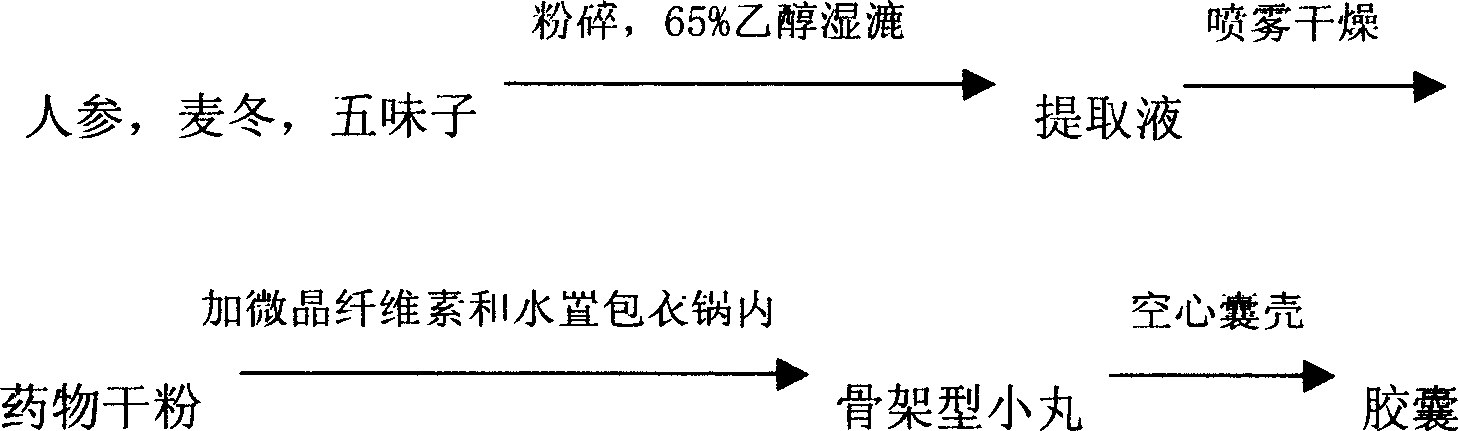
[0037] Prescription: 100g of ginseng, 200g of Ophiopogon japonicus, 100g of schisandra, 100g of microcrystalline cellulose, hollow capsules
[0038] 65% ethanol solution
[0039] Preparation method:
[0040] The process route is as follows
[0041]
[0042] The ginseng, Ophiopogon and Schisandra are washed, crushed, and soaked in a 65% ethanol solution for 24 hours, and then subjected to wet treatment. The extract is spray-dried at 5-7 ml / min to obtain a dry powder of the medicine. Put the microcrystalline cellulose and the drug dry powder into a coating pan with a rotation speed of 15 r / min, and obtain 500 g of dry pellets at 40° C. and 10 r / min. Put it into capsules to get 1000 capsules.
[0043] After testing, it contains ginsenoside Rg 1 The total content of Re is 0.25%, the total content of Ophiopogon saponins B and D accounts for 0.25%, and the content of Schisandrin A accounts for 0.30%.
Embodiment 2
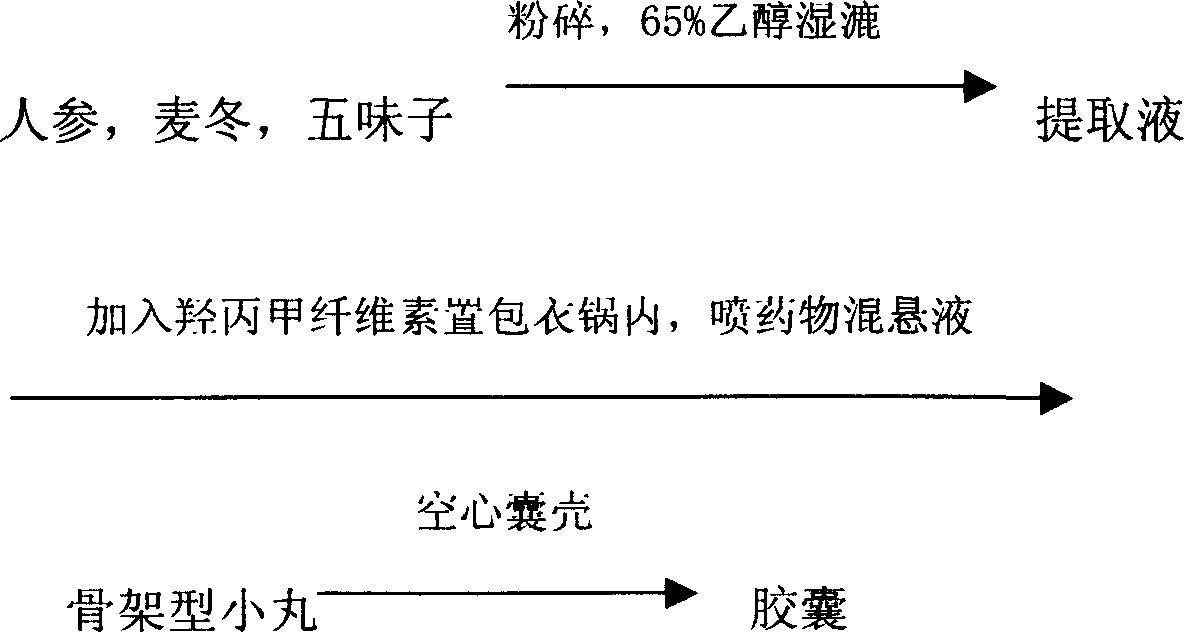
[0045] Prescription: 100g ginseng, 200g Ophiopogon japonicus, 100g schisandra, 120g hypromellose, hollow capsule, 65% ethanol solution
[0046] Preparation method:
[0047] The process route is as follows
[0048]
[0049] The ginseng, Ophiopogon and Schisandra are washed, crushed, soaked in a 65% ethanol solution for 24 hours and then subjected to wet treatment to obtain an extract. Put the hypromellose into the coating pan, spray the extract at a rate of 5-7ml / min at 40°C, and dry 500g of skeleton-shaped pellets. Put it into capsules to get 1000 capsules.
[0050] After testing, it contains ginsenoside Rg 1 The total content of Re accounted for 0.25%, the total content of Ophiopogon saponins B and D accounted for 0.35%, and the content of Schisandrin A accounted for 0.30%.
Embodiment 3
[0052] Using the process of preparing the matrix pellets in Example 1 or 2, different sustained-release materials such as methyl cellulose (MC), hypromellose (HPMC), povidone (PVP), ethyl cellulose, cellulose acetate , Polymethacrylate 80-100g, prepare 500g skeleton-shaped pellets, and then put them into capsules to obtain 1000 capsules. The test results are basically the same as those of Examples 1 and 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com