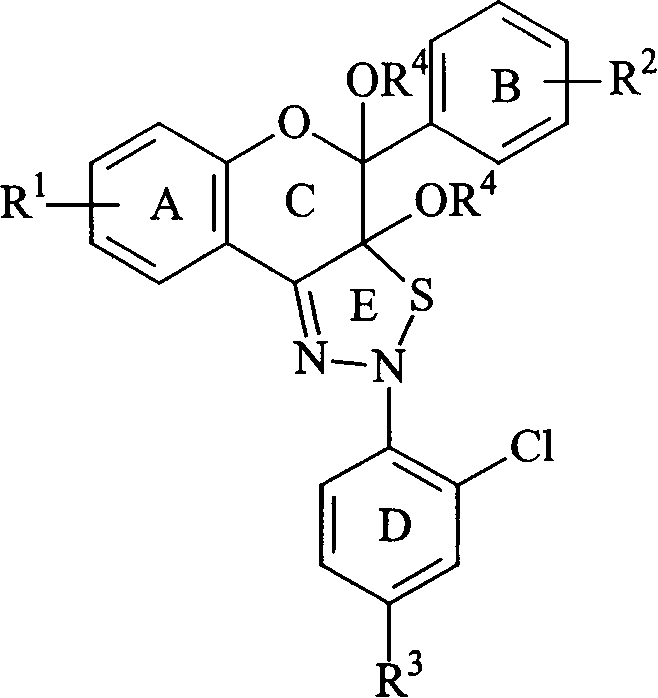
Poly-substituted flavan zothia dizoline kind compound and preparation process and use thereof
A technology of thiadiazolines and compounds, applied in the field of synthesis of organic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of flavanone-4-(4-nitrophenyl) hydrazone (compound IVa)
[0025] Add 0.448g (2mmol) of flavanone, 0.306g (2mmol) of 4-nitrophenylhydrazine, 20mL of ethanol and 0.02mL of acetic acid into the reaction flask, and reflux for 6hr. After the solid dissolves, a yellow solid precipitates out. Cool, filter with suction, wash the filter cake with a small amount of ethanol, and dry under reduced pressure to obtain 0.626 g of a yellow solid (Compound IVa), with a yield of 87.2%. Melting point: 249-250°C.
[0026] 1 H NMR (δ, DMSO-d 6): 2.77-2.84(m, 1H), 3.35-3.40(m, 1H), 5.25(dd, 1H, J=12.0Hz, 3.2Hz), 6.96(d, 1H, J=8.4Hz), 7.03(t , 1H, J=8.0, 16.0Hz), 7.27(t, 1H, J=8.0, 16.0Hz), 7.35(m, 2H), 7.38(d, 1H, J=7.2Hz), 7.44(t, 2H, J=7.2, 14.4Hz), 7.56(d, 2H, J=7.2Hz), 8.07(d, 1H, J=8.0Hz), 8.11(d, 2H, J=8.8Hz), 10.31(s, 1H) .
Embodiment 2
[0027] Example 2: Preparation of 4'-methoxyflavanone-4-(4-nitrophenyl)hydrazone (compound IVb)
[0028] The operation process is the same as in Example 1, except that flavanone is replaced by 4'-methoxyflavanone. A golden yellow solid (compound IVb) was obtained with a yield of 93.2%. Melting point: 237-238°C.
[0029] 1 H NMR (δ, DMSO-d 6 ): 2.80-2.87(m, 1H), 3.29-3.34(m, 1H), 3.75(s, 3H), 5.18(dd, 1H, J=12.0Hz, 2.8Hz), 6.93(d, 1H, J= 8.0Hz), 6.98(d, 2H, J=8.8Hz), 7.27(t, 1H, J=8.0, 16.0Hz), 7.32(m, 2H), 7.47(d, 2H, J=8.8Hz), 7.56 (d, 2H, J = 7.2Hz), 8.06 (d, 1H, J = 7.6Hz), 8.11 (d, 2H, J = 7.6Hz), 10.31 (s, 1H).
Embodiment 3
[0030] Example 3: Preparation of 5,7-dimethoxyflavanone-4-(4-nitrophenyl)hydrazone (compound IVc)
[0031] The operation process is the same as in Example 1, except that flavanone is replaced by 5,7-dimethoxyflavanone. An orange-red solid (compound IVc) was obtained with a yield of 89.3%. Melting point: 214-216°C.
[0032] 1 H NMR (δ, DMSO-d 6 ): 2.73-2.80(m, 1H), 3.30-3.35(m, 1H), 3.77(s, 3H), 3.92(s, 3H), 5.18(dd, 1H, J=12.0Hz, 3.2Hz), 6.23 (d, 1H, J = 2.4Hz), 6.30 (d, 1H, J = 2.4Hz), 7.29 (d, 2H, J = 2.4Hz), 7.39 (t, 1H, J = 7.2, 14.4Hz), 7.46 (t, 2H, J=7.2, 14.4Hz), 7.56(d, 2H, J=8.0Hz), 8.13(d, 2H, J=8.0Hz), 10.12(s, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com