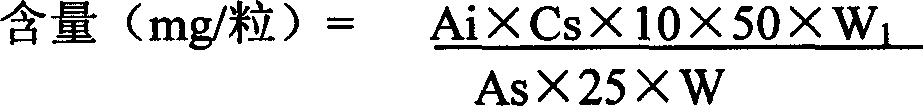Method for quality control of Qianbai biyan solid prepn. for treating rhinitis
A quality control method and solid preparation technology, which is applied in the field of Qianbai rhinitis solid preparation made of 16g of Angelica dahurica and 16g of Chuanxiong, notopterygium 32g, which can solve the problems of large limitations, difficulties in production and management, and difficulty in controlling the quality of Qianbai rhinitis tablets, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] The quality control of embodiment 1 capsules of the present invention
[0142] Take Chinese medicinal materials Senecio 4848g, Selaginella 808g, Cassia 484g, Ephedra 162g, Notopterygium 32g, Angelica dahurica 16g and Ligusticum chuanxiong. liquid, filtered, and the filtrate was concentrated into a thick ointment; the rest of notopterygium and other shamisen were crushed into fine powder, added to the above thick ointment, stirred, dried, pulverized into fine powder, mixed evenly, packed into capsules, and made into 1000 capsules.
[0143] 1, measure the content of ephedrine with high performance liquid chromatography (an appendix VI D of Chinese Pharmacopoeia version in 2000):
[0144] Test equipment, reagents, reagents
[0145] Shimadzu LC-10ATvp high performance liquid chromatograph, LC-10ATvp infusion pump; SPD-M10Avp secondary array detector; SCL-10Avp system controller; CTO-10ASvp constant temperature column oven; CLASS-VP6.1 workstation data processing system; M...
Embodiment 2
[0171] The quality control of embodiment 2 tablet of the present invention
[0172] Take Chinese medicinal materials Senecio 4848g, Selaginella 808g, Cassia 484g, Ephedra 162g, Notopterygium 32g, Angelica dahurica 16g and Ligusticum chuanxiong. liquid, filtered, and the filtrate was concentrated into a thick ointment; the remaining notopterygium and other shamisen were crushed into fine powder, added to the above thick ointment, stirred evenly, dried, pulverized into fine powder, mixed evenly, added appropriate amount of auxiliary materials, and pressed into tablets to make 1000 tablets. Instantly.
[0173] 1, measure the content of ephedrine with high performance liquid chromatography (an appendix VI D of Chinese Pharmacopoeia edition in 2000):
[0174] Test equipment, reagents, reagents
[0175] Shimadzu LC-10ATvp high performance liquid chromatograph, LC-10ATvp infusion pump; SPD-M10Avp secondary array detector; SCL-10Avp system controller; CTO-10ASvp constant temperature c...
Embodiment 3
[0201] The quality control of embodiment 3 granules of the present invention
[0202] Take Chinese medicinal materials Senecio 4848g, Selaginella 808g, Cassia 484g, Ephedra 162g, Notopterygium 32g, Angelica dahurica 16g and Ligusticum chuanxiong. liquid, filtered, and the filtrate was concentrated into a thick ointment; the remaining notopterygium and other shamisen were crushed into fine powder, added to the above thick ointment, stirred evenly, granulated, dried, granulated, mixed evenly, and packed separately, each bag had a capacity of 10g, and the product was obtained.
[0203] 1, measure the content of ephedrine with high performance liquid chromatography (an appendix VI D of Chinese Pharmacopoeia edition in 2000):
[0204] Test equipment, reagents, reagents
[0205] Shimadzu LC-10ATvp high performance liquid chromatograph, LC-10ATvp infusion pump; SPD-M10Avp secondary array detector; SCL-10Avp system controller; CTO-10ASvp constant temperature column oven; CLASS-VP6.1 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com