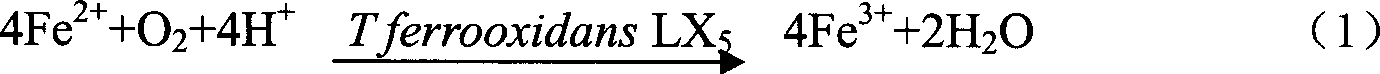Biosynthesis of obligate adsorbent and its usage in adsorbing to eliminate As and Cr from water
A technology of microorganisms and deionized water, applied in the field of environmental engineering, can solve the problem that the adsorption capacity and selective adsorption capacity cannot be combined, and achieve the effect of small particles, large specific surface area and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The biosynthesis of embodiment 1Schwertmannite:
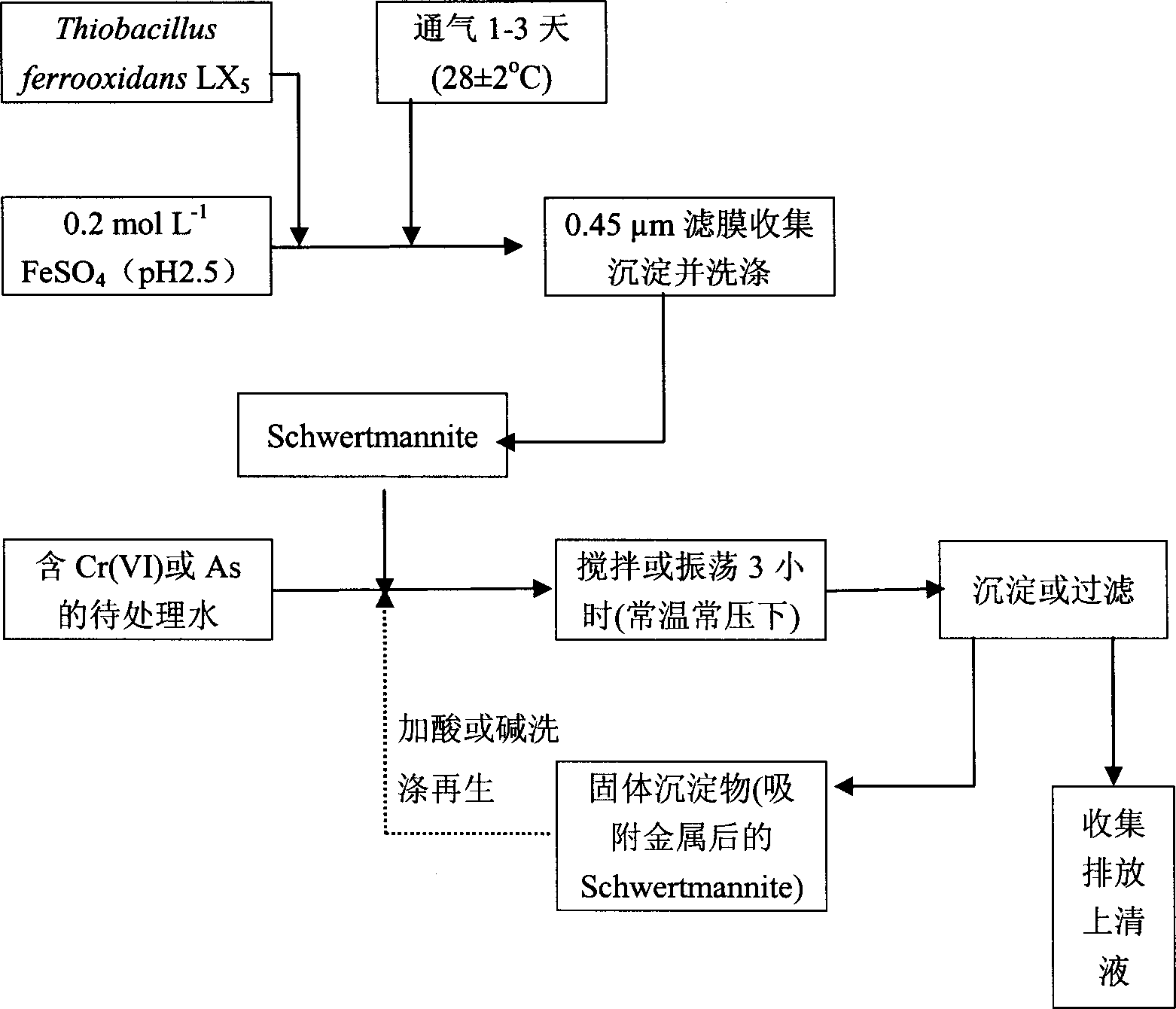
[0030] Add 6.95kg FeSO to the reactor 4 ·7H 2 O and 250L deionized water, stirring to dissolve the iron salt and adjusting the pH to 2.5 with sulfuric acid, inoculated with Thiobacillus ferrooxidans LX 5 ), so that its number reaches 10 7 ~10 8 per ml, under normal temperature and pressure (28±2°C and one standard atmospheric pressure), ventilate and stir for 1-3 days. The precipitate was collected by filtration, washed with deionized water acidified with sulfuric acid (pH1.0-2.5) for 2-3 times, and then rinsed with deionized water for 2-3 times until there was no SO in the filtered filtrate. 4 2- until. Bake at 55-80° C. for 2-3 hours to obtain 1.29 kg of dry product (the product is reddish-brown amorphous solid particles), with a yield of 54%.
[0031] The present invention relates to the microorganisms used to catalyze the oxidation synthesis of Schwertmannite is Thiobacillus ferrooxidans LX 5 ), which is a st...
Embodiment 2
[0033] Example 2 Schwertmannite Adsorption Removal of Cr(VI) in Wastewater
[0034] (1) Operation steps: add the adsorbent Schwertmannite to 5-1000mgCr(VI) / L wastewater according to 0.5% of the treated water, and use 0.2-1.0mol / L dilute HNO 3 or NaOH to adjust the pH value of the above-mentioned Cr(VI) wastewater containing adsorbent Schwertmannite to 7.0, oscillate (180r / min) or stir at constant temperature and pressure (28±2°C and 1 standard atmospheric pressure) or stir for 3h, take a sample and centrifuge, filter, and measure Filtrate pH value and Cr (VI) concentration.
[0035] (2) Analysis of results: The pH value was measured with a precision digital pH meter [PHS-2TC (0.01)], and the Cr(VI) was measured with a diphenylcarbazide colorimetric method. According to the change of Cr(VI) concentration in the solution before and after the experiment, the removal rate and maximum adsorption capacity of Cr(VI) were calculated. After analysis and calculation, the maximum capaci...
Embodiment 3
[0037] Example 3 Schwertmannite adsorption removes Cr(VI) in wastewater where multiple ions coexist
[0038] (1) The influence of coexisting heavy metal ions on the adsorption and removal of Cr(VI):
[0039] Add the adsorbent Schwertmannite to the Zn containing 50mg / L according to 0.5% of the treated water 2+ 、Cu 2+ 、Cd 2+ And in electroplating wastewater with a concentration of 10-200mg / L Cr(VI), the main anion is SO 4 2- . Use 0.2~1.0mol / L dilute HNO 3 or NaOH to adjust the pH value of the wastewater containing the adsorbent Schwertmannite to 5.0, oscillate (rotate at 180r / min) or stir for 3h at constant temperature and pressure (28±2°C and 1 standard atmospheric pressure), sample and centrifugally filter, and use PE3100 atomic absorption spectrometry Photometric determination of Zn 2+ 、Cu 2+ 、Cd 2+ The Cr(VI) content was determined by the diphenylcarbazide colorimetric method, and the adsorption removal rate and adsorption amount of heavy metals were calculated.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com