Novel vanadium halide redox flow battery
A vanadium halide, battery technology, applied in indirect fuel cells and other directions, can solve problems such as reducing the life of battery components, bromine gas leakage, and increasing corrosion characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0110] Throughout the specification and claims the term polyhalide complex or ion is a complex or ion of three or more halogen atoms. The polyhalide complex is Br 3 - , ClBr 2 - and BrCl 2 - (See PCT / AU02 / 01157, which is hereby incorporated by cross-reference for further exemplary purposes).
[0111] Throughout the specification, the terms electrolyte and supporting electrolyte are used interchangeably. The electrolyte used in the redox battery of the present invention is preferably an aqueous electrolyte.
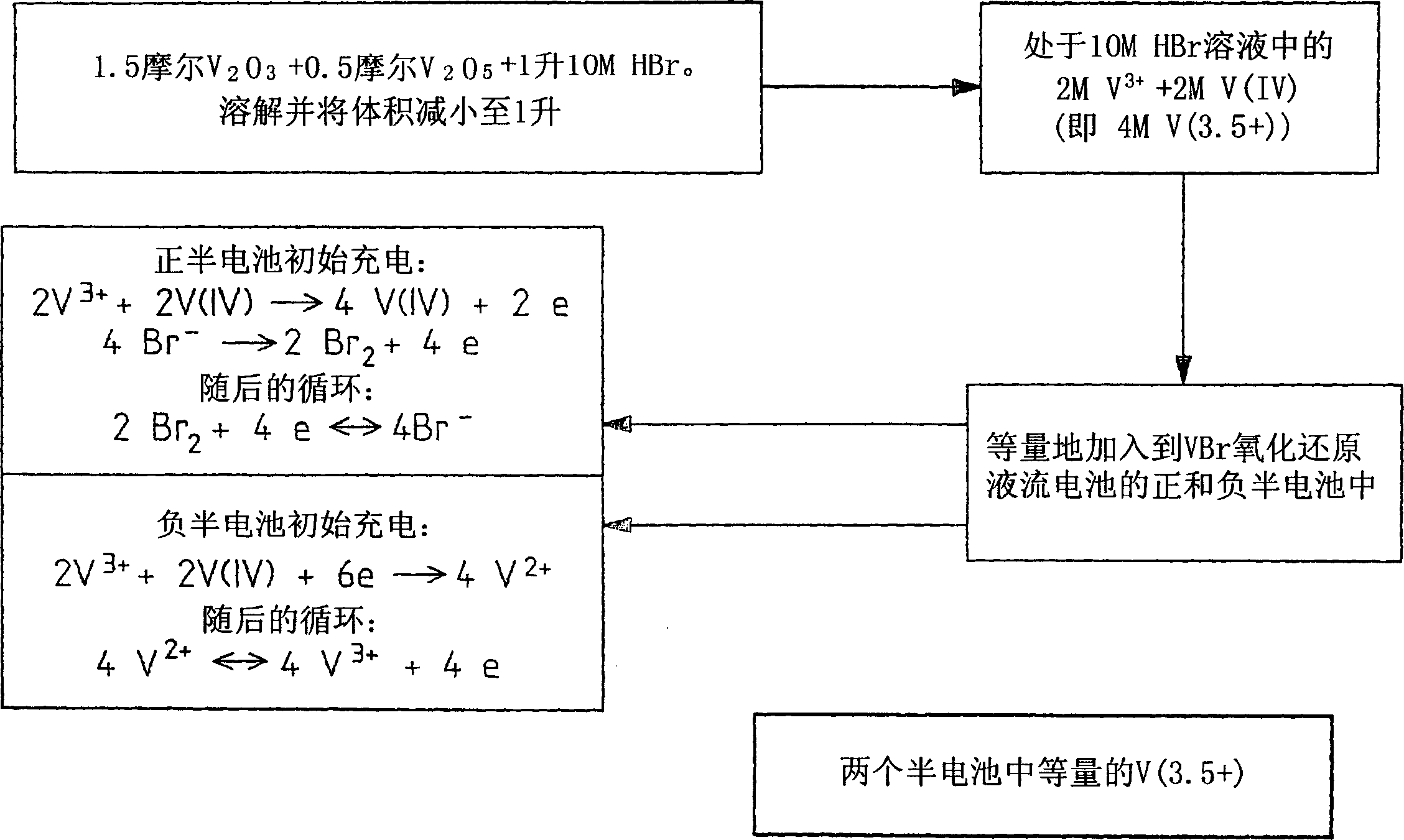
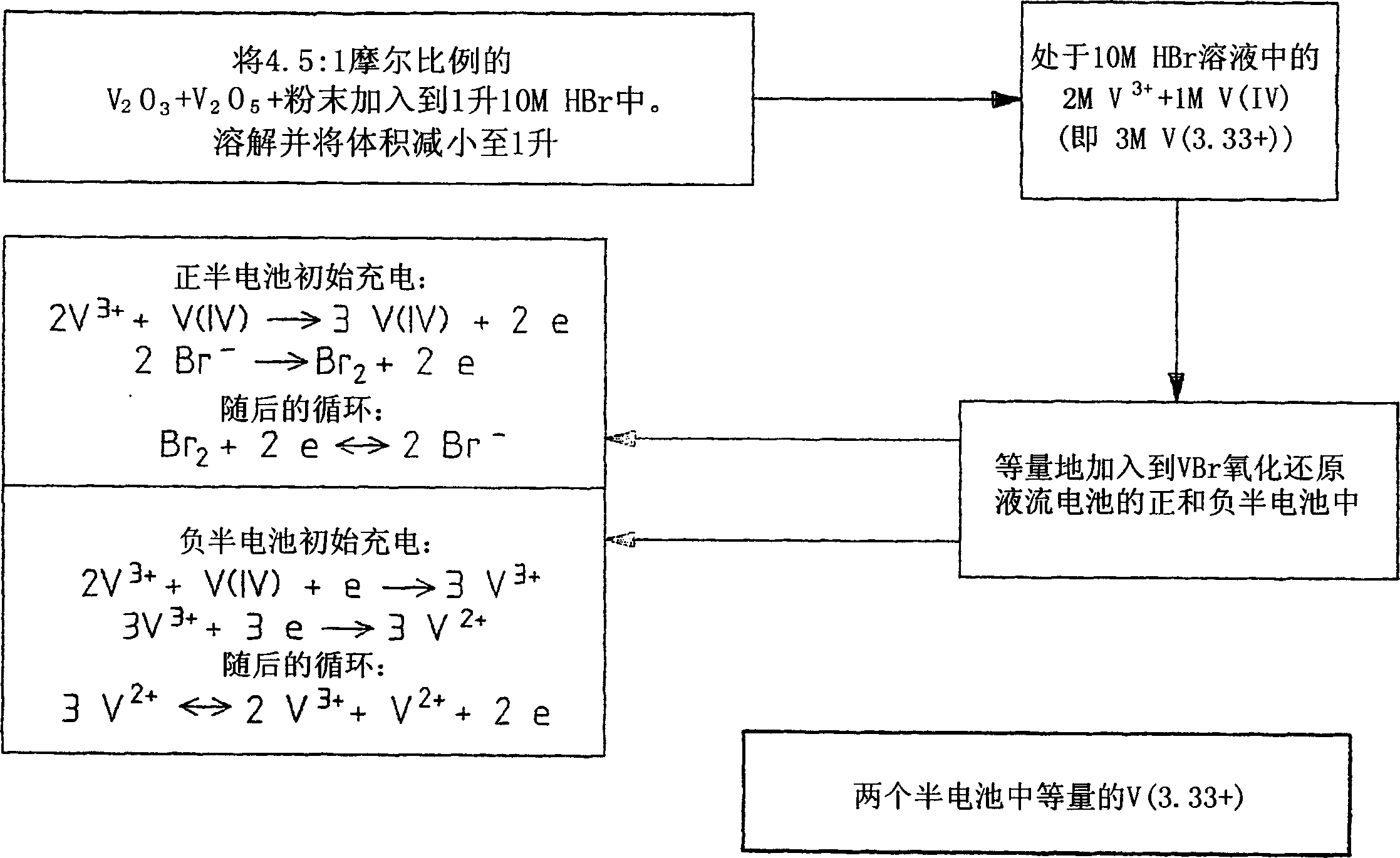
[0112] In the modified vanadium halide redox flow battery, a 50:50 mixture solution of the halides V(IV) and V(III) (referred to as V(3.5+)) was used as the initial feedstock for the positive and negative half-cells. electrolytic solution. Thus, in contrast to brominated redox flow batteries employing brominated V(IV) feed solutions, during the initial charge of the modified battery, the V(III) and V(IV) ions in the negative half-cell react according to is reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com