Gallium nitrate preparation method
A technology of gallium nitrate and nitric acid, applied in chemical instruments and methods, gallium/indium/thallium compounds, inorganic chemistry, etc., can solve problems such as long crystallization time, difficult crystallization, and difficult dissolution, so as to accelerate the speed of product precipitation, The effect of shortening the induction period and shortening the seed precipitation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
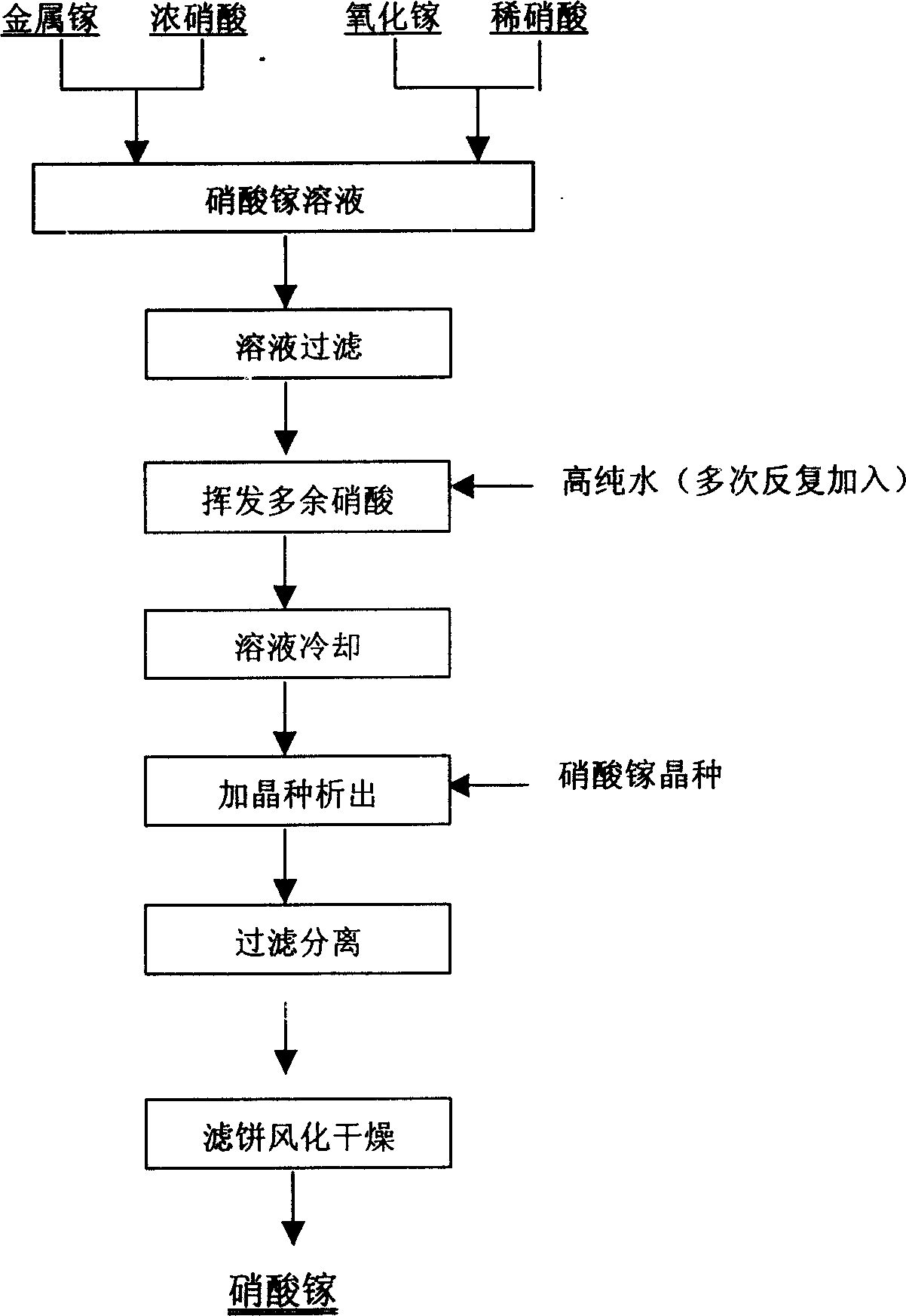
[0014] Dissolve metal gallium in hot concentrated nitric acid at 50°C. After the metal gallium is completely dissolved, evaporate the solution, keep the reaction temperature at 100°C, add water after concentration, repeat concentration and evaporation until there is no smell of nitric acid in the solution, cool the solution until -20°C, add seed crystals at the same time, the weight ratio of the added seed crystals to the weight of gallium nitrate in the solution, that is, the seed crystal coefficient is 0.03, carry out stirring and decomposition of the seeds, and obtain hydrated gallium nitrate crystals after separation, and air-dry at 30°C Within 2 days, using ICP-MS analysis and detection technology, the product quality can reach more than 99.99%.
Embodiment 2
[0016] Dissolve metal gallium in hot concentrated nitric acid at 65°C. After the metal gallium is completely dissolved, evaporate the solution, keep the reaction temperature at about 90°C, control the solution to slightly boil, after concentration, add water, and concentrate until there is no smell of nitric acid in the solution. Cool the solution to 0°C, add seed crystals (the seed crystal coefficient is 0.01) at the same time to stir and decompose the seeds, and obtain hydrated gallium nitrate crystals after separation, air-dry at 40°C for 1 day, use ICP-MS analysis and detection technology, product quality It can reach more than 99.99%.
Embodiment 3
[0018] Use high-purity water and high-purity reagents for raw material preparation, and control the appropriate acid-soluble and crystallization conditions: dissolve metal gallium in hot concentrated nitric acid at 85°C, wait until the metal gallium is completely dissolved, evaporate the solution, maintain the reaction temperature at 80°C, and concentrate Finally, add water, concentrate until there is no nitric acid odor in the solution, cool the solution to -10°C, and add seed crystals (the seed crystal coefficient is 0.05) at the same time to carry out stirring and decomposition of the seeds, and the hydrated gallium nitrate crystals can be obtained after separation. Air-dried at 40°C for 2 days, using ICP-MS analysis and detection technology, the product quality can reach more than 99.99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com