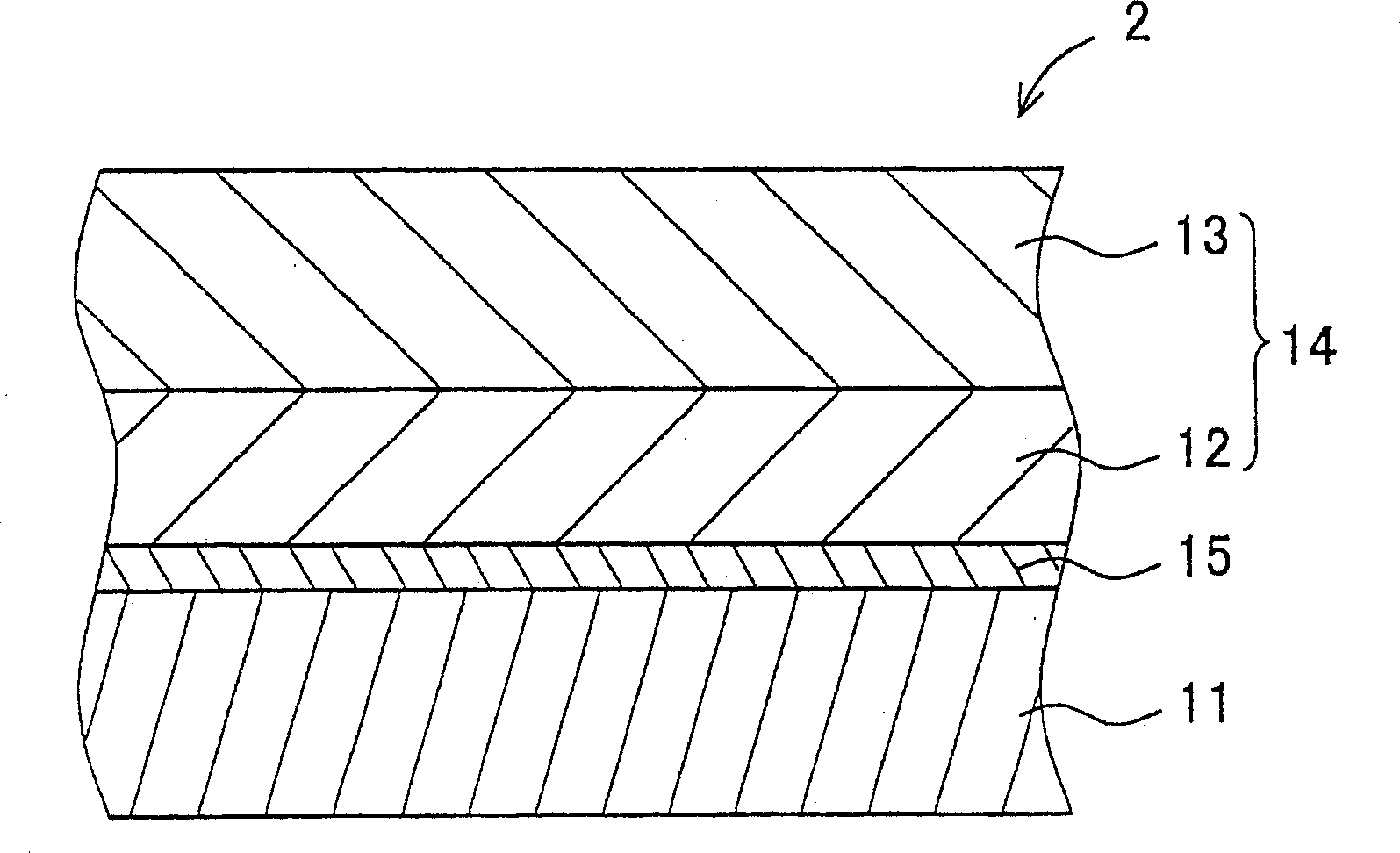
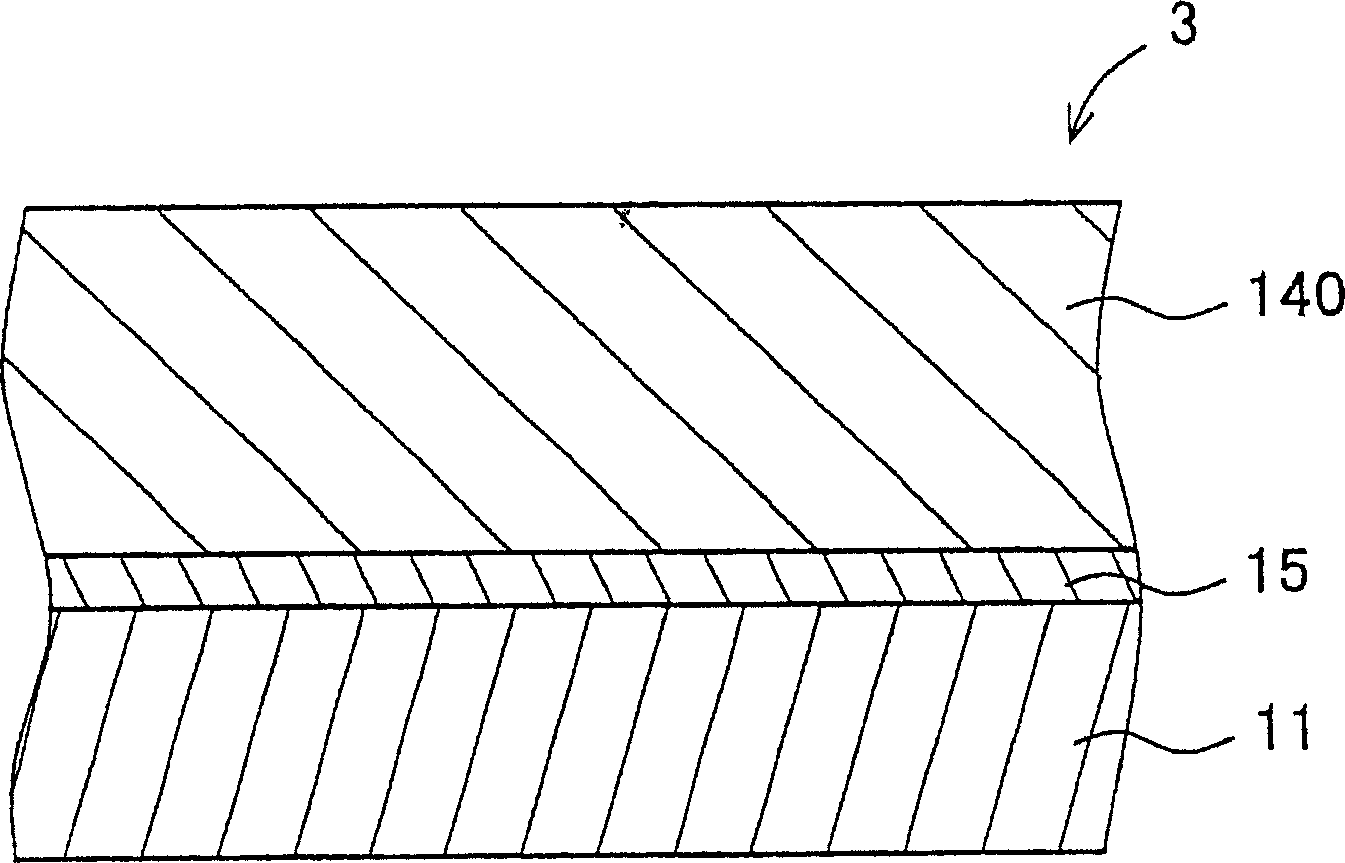
Hydrazone compound, electrophotographic photoreceptor comprising the hydrazone compound, and image forming apparatus equipped with the electrophotographic photoreceptor
An electrophotography and compound technology, which is applied to the equipment of the electric recording process applying the charge pattern, the electric recording process applying the charge pattern, optics, etc., to achieve improved sensitivity and resolution, excellent electrical characteristics, and excellent environmental stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0239] (Production Example 1) Production of Exemplary Compound No.1
[0240] Use acetophenone as the ketone compound represented by the above general formula (4a), use N,N-diphenylhydrazine as the hydrazine compound represented by the general formula (4b), carry out the dehydration condensation reaction as described below, and produce the following structural formula ( 8) The hydrazone intermediate represented.
[0241]
[0242] 15.1 g (1.0 molar equivalent) of p-methoxyacetophenone, 19.5 g (1.05 molar equivalent) of N,N-diphenylhydrazine, and 0.06 mL (0.01 molar equivalent) of acetic acid as a catalyst were added to 100 mL of ethanol, and heated to 80 degreeC, stirring for 4 hours, it was made to react. After cooling the reaction solution, 100 mL of hexane was added thereto, and the precipitated crystals were collected by filtration and dried under reduced pressure to obtain 27.8 g (yield 88.0%) of the hydrazone intermediate represented by structural formula (8) as yellow...
manufacture example 2
[0255] (Production Example 2) Production of Exemplary Compound No.18
[0256] In Production Example 1, during the Grignard reaction, 2.2 g (1.2 molar equivalents) of 5-bromo-1-phenyl-1,3-pentadiene represented by the following structural formula (11) was used instead of structural formula (10) Except for the cinnamyl bromide (1.2 molar equivalents), 3.30 g of a yellow powdery compound was obtained in the same manner as in Production Example 1.
[0257]
[0258] The obtained compound was analyzed by LC-MS. As a result, a hydrazone-hexatriene compound (calculated molecular weight: 484.25) corresponding to the target compound, the hydrazone-hexatriene compound (calculated molecular weight: 484.25) corresponding to the exemplary compound No. 18 shown in Table 1, was observed at 485.4. Molecular ion [M+H] + peak. Therefore, it was confirmed that the obtained compound was a hydrazone-hexatriene compound of Exemplary Compound No. 18 (yield: 81.0%).
[0259]In addition, the puri...
manufacture example 3
[0263] (Production Example 3) Production of Exemplary Compound No.28
[0264] In Production Example 1, during the dehydration condensation reaction, 3,4-methylenedioxybenzaldehyde was used instead of p-methoxyacetophenone, and the same operation was performed to obtain the hydrazone intermediate represented by the following structural formula (12) , as yellow crystals (98% yield).
[0265]
[0266] Then, 9.2 g (1.2 molar equivalents) of phosphorus oxychloride was slowly added to 100 mL of anhydrous N,N-dimethylformamide (abbreviated as DMF) under ice-cooling, and stirred for about 30 minutes to prepare a Wellsmeyer reagent. To this solution, 15.82 g (1.0 molar equivalent) of the hydrazone intermediate represented by the structural formula (12) obtained above was slowly added under ice-cooling. Then, heating was gradually performed, the temperature of the reaction solution was raised to 70° C., and the mixture was stirred for 3 hours while maintaining heating at 70° C., to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com