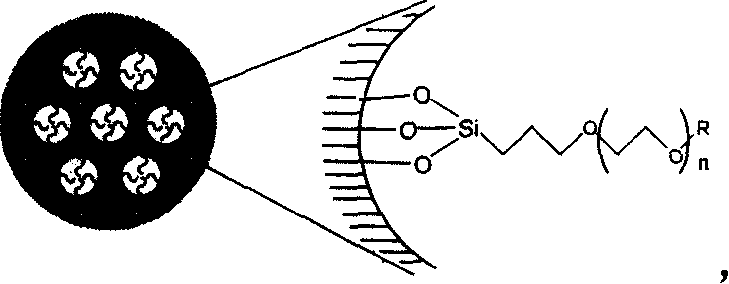
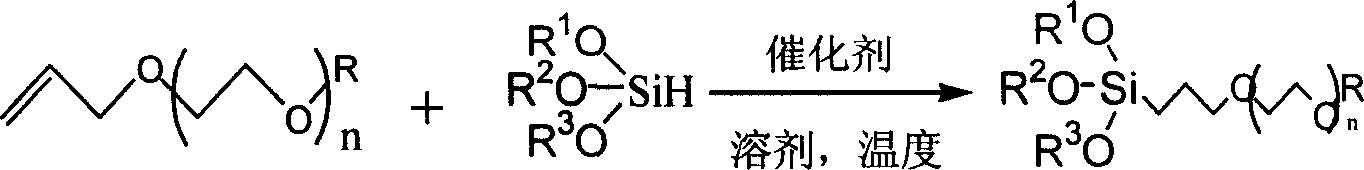
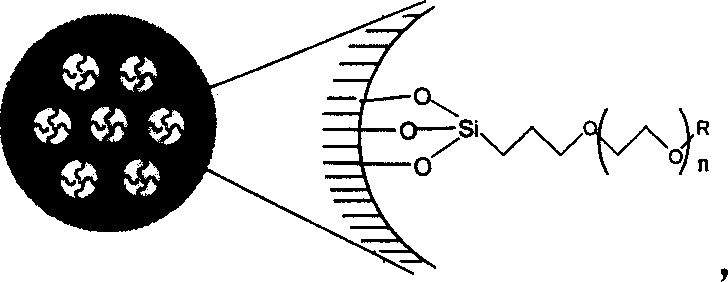
Compound, synthetic method and application of organosilicon containing carbon-oxygen-ether linkage
A technology of organosilicon compounds and carbon-containing oxyethers, applied in the directions of organic compounds/hydrides/coordination complex catalysts, organic chemistry, chemical instruments and methods, etc. Simple preparation and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of organosilicon compound 1:
[0026]
[0027] The operation is as follows: chloroplatinic acid (0.5mol%) was vacuum-dried at 180°C for 2 hours, added ethyl-allyl-tetraethylene glycol ether (0.1mol), triethoxysilyl hydrogen (0.3mol), and stirred at room temperature 1 day. Excess triethoxysilane was distilled off, the vacuum pump depressurized, and the colorless liquid was distilled off at 178°C to obtain 27.5 g of the product. 1 H NMR (300MHz, CDCl 3 ): δ3.79(q, J=7.2Hz, 6H), 3.64-3.43(m, 18H), 3.39(t, J=6.9Hz, 2H), 1.71-1.61(m, 2H), 1.19-1.14( m, 12H), 0.59 (t, J=8.4Hz, 2H). 13 C NMR (75MHz, CDCl 3 ): δ73.4, 70.9, 70.6, 70.1, 63.0, 51.3, 17.9, 15.5, 14.7, 8.0; MS m / z (%) 337 (100), 427 (M + , 35.45); Elemental analysis calculated value, C 19 h 42 o 8 Si: C, 53.49; H, 9.92; Found: C, 53.65; H, 9.70.
Embodiment 2
[0029] (2) Preparation of organosilicon compound 2:
[0030]
[0031] The operation is as follows: the operation is as follows: chloroplatinic acid (0.5mol%) was vacuum-dried at 180° C. for 2 hours, added methyl-allyl-tetraethylene glycol ether (0.1mol), triethoxysilyl hydrogen (0.3mol) , stirred at room temperature for 1 day. Excess triethoxysilane was distilled off, the vacuum pump depressurized, and the colorless liquid was distilled off at 178°C to obtain 27.3 g of the product. 1 H NMR (300MHz, CDCl 3 ): δ3.78(q, J=7.2Hz, 6H), 3.64-3.43(m, 18H), 3.39(t, J=6.9Hz, 2H), 3.25(t, J=8.4Hz, 3H), 1.71 -1.61 (m, 2H), 1.23 (t, J=8.2Hz, 9H), 0.59 (t, J=8.4Hz, 2H). 13 C NMR (75MHz, CDCl 3 ): δ70.9, 70.6, 70.1, 63.0, 53.9, 51.3, 17.9, 15.5, 8.0; MS m / z (%) 323 (100), 413 (M + , 22.23); Elemental analysis calculated value, C 18 h 40 o 8 Si: C, 52.40; H, 9.77; Found: C, 52.62; H, 9.65.
Embodiment 3
[0033] (3) Preparation of organosilicon compound 3:
[0034]
[0035] The operation is as follows: chloroplatinic acid (0.5mol%) was vacuum-dried at 180°C for 2 hours, added phenyl-allyl-tetraethylene glycol ether (0.1mol), triethoxysilyl hydrogen (0.3mol), and stirred at room temperature 1 day. Excess triethoxysilane was distilled off, the vacuum pump depressurized, and the colorless liquid was distilled off at 178°C to obtain 32.8g of the product. 1 H NMR (300MHz, CDCl 3 ): δ6.90-6.76(m, 5H), 4.20(t, J=6.9Hz, 2H), 3.79(q, J=7.2Hz, 6H), 3.64-3.43(m, 16H), 1.70-1.64( m, 2H), 1.19-1.14 ((t, J = 7.2Hz, 9H), 0.69 (t, J = 8.4Hz, 2H). 13 C NMR (75MHz, CDCl 3 ): δ158.8, 129.1, 120.1, 114.2, 73.4, 70.9, 70.6, 70.5, 51.3, 17.9, 15.5, 14.7, 8.0; MS m / z (%) 385 (100), 475 (M + , 22.54); Elemental analysis calculated value, C 23 h 42 o 8 Si: C, 58.20; H, 8.92; Found: C, 58.45; H, 8.70.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com