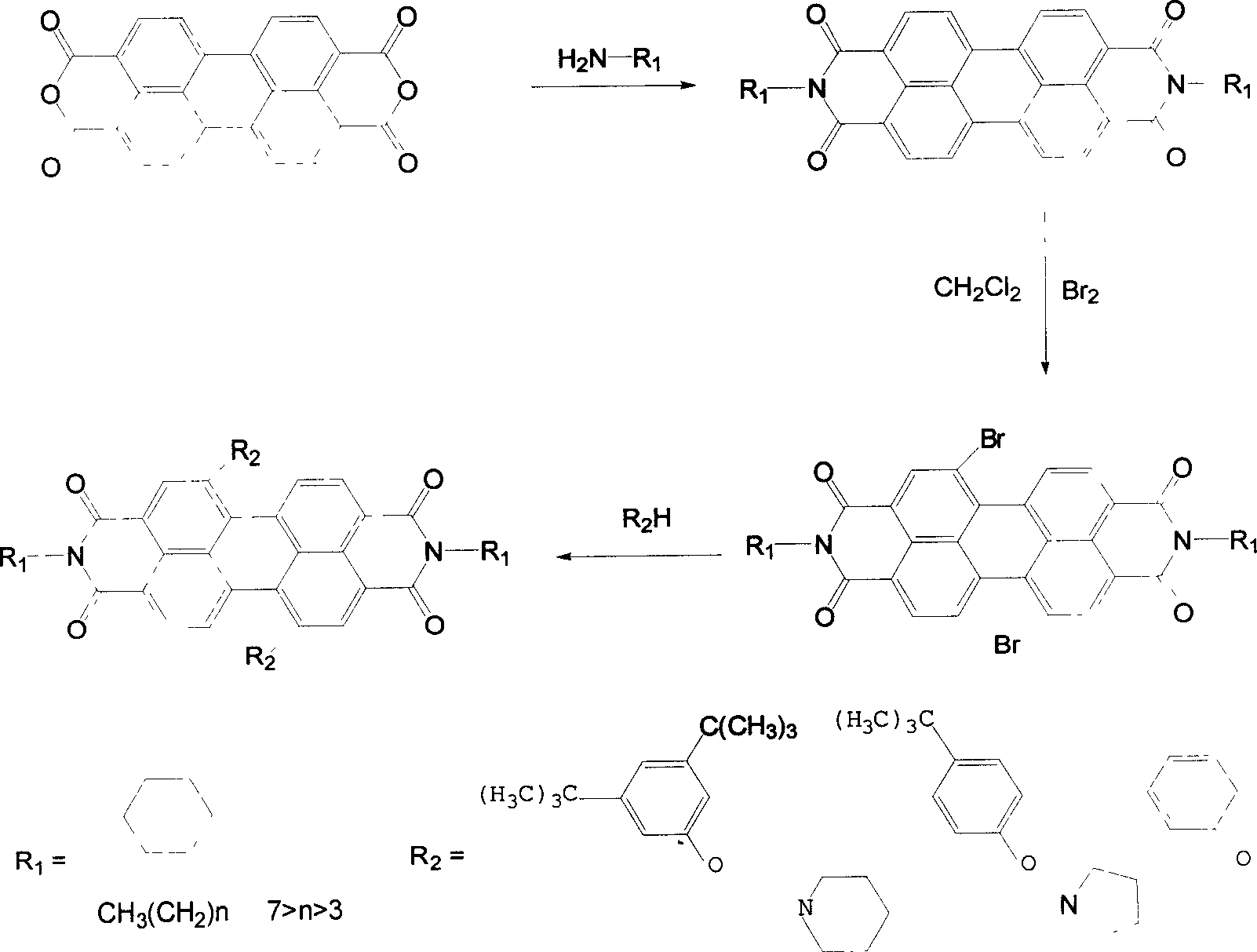
Synthesis process of 1,7-disubstituent-3,4:9,10-perylene bisimide
A technology of perylenebisimide and dibromoperylenediimide, which is applied in the field of organic chemical industry and fine chemical industry, can solve the problems of low substitution reaction yield, difficulty in increasing yield, and many by-products, and shorten the reaction time. time, increased productivity, and low cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
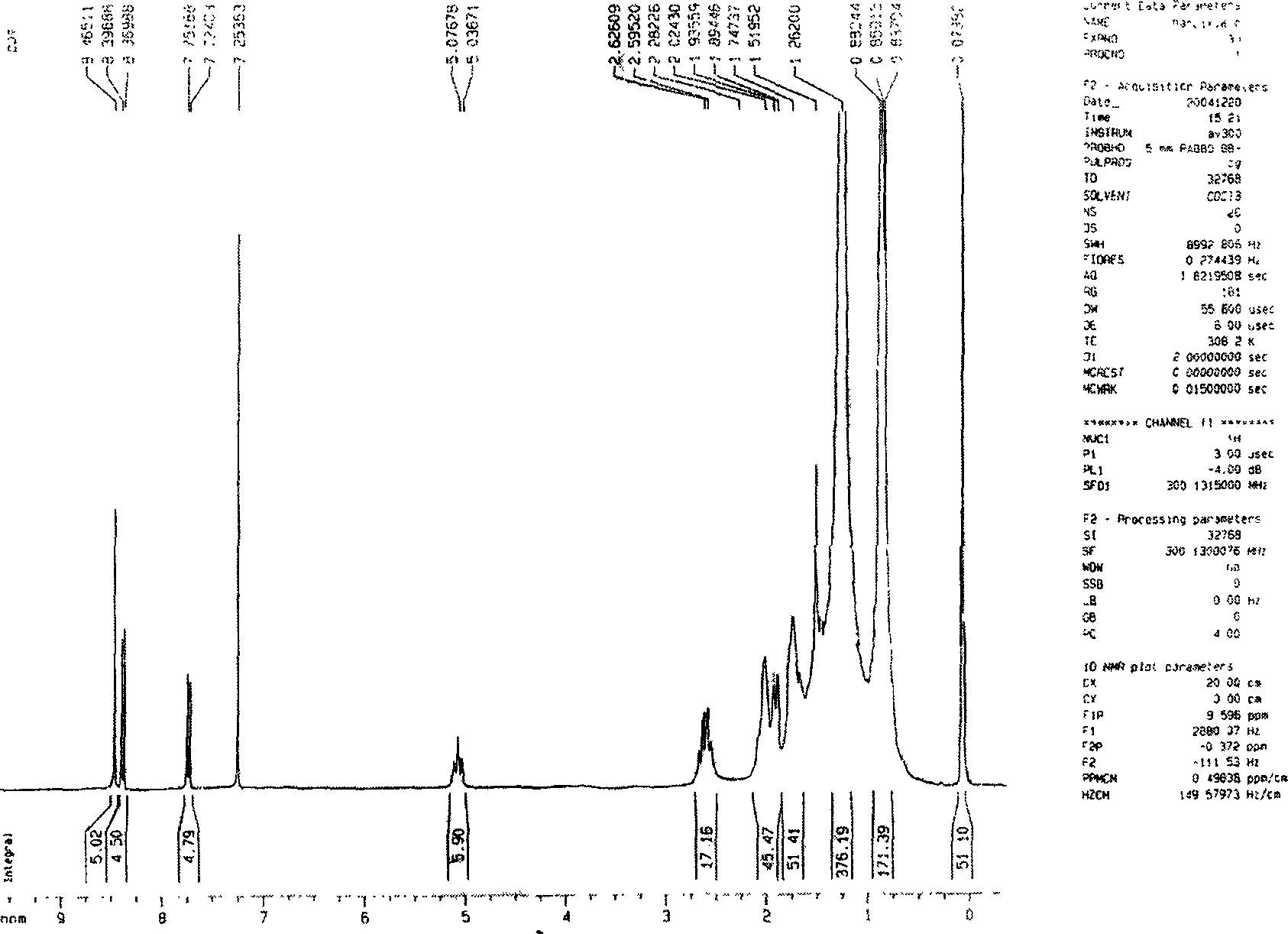
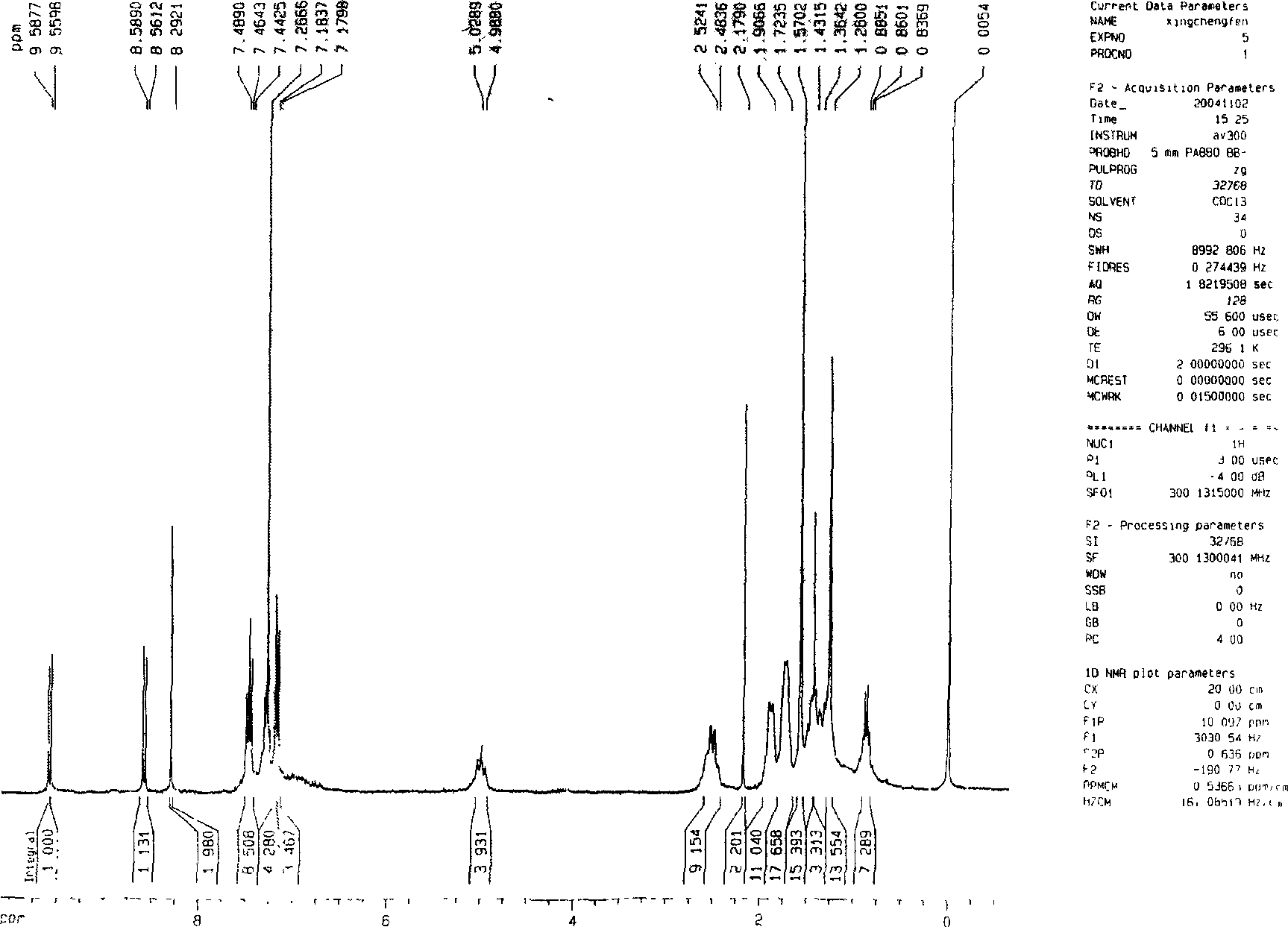
[0019] Add 10 grams of perylenetetracarboxylic dianhydride and 300 milliliters of cyclohexylamine into a 500 milliliter single-necked round-bottomed flask, reflux at 135° C. for 30 hours, and track the progress of the reaction with a thin-layer silica gel plate. Excess cyclohexylamine was distilled off and recovered to generate perylene diimide with a yield of 100%. Perylene diimide and liquid bromine were refluxed for 40 hours at 40°C under the protection of argon in the solvent dichloromethane, and the concentration of bromine was controlled to be 20 g / 100 ml of dichloromethane, and the amount of dichloromethane added was 100 Milliliters per gram of perylene diimide, after the reaction is complete, the mixed solution of dichloromethane and liquid bromine is distilled off at normal pressure and recovered to generate dibromoperylene diimide with a yield of 95%. Add 1.2 grams of dibromoperylene diimide, 0.54 grams of p-tert-butylphenol, 0.48 grams of potassium carbonate and 20 ...
Embodiment 2
[0021] Add 10 grams of perylenetetracarboxylic dianhydride and 400 milliliters of n-butylamine into a 500 milliliter single-necked round bottom flask, reflux at 80°C for 60 hours, and track the progress of the reaction with a thin-layer chromatography silica gel plate. Excess n-butylamine was distilled off and recovered to generate perylene diimide with a yield of 100%. Perylene diimide and liquid bromine were refluxed for 60 hours at 40°C under the protection of argon in the solvent dichloromethane, and the concentration of bromine was controlled to be 10 g / 100 ml of dichloromethane, and the amount of dichloromethane added was 30 Milliliters per gram of perylene diimide, after the reaction is complete, the mixed solution of dichloromethane and liquid bromine is evaporated under normal pressure and recovered to generate dibromoperylene diimide with a yield of 85%. Add 1.2 grams of dibromoperylene diimide, 0.32 grams of phenol, 0.45 grams of sodium carbonate, and 20 milliliters...
Embodiment 3
[0023] Add 10 grams of perylenetetracarboxylic dianhydride and 350 milliliters of n-pentylamine into a 500 milliliter single-necked round-bottomed flask, and reflux at 105° C. for 50 hours. The progress of the reaction was followed by a thin-layer chromatography silica gel plate. Excessive n-pentylamine was distilled off and recovered to generate perylene diimide with a yield of 100%. Perylene diimide and liquid bromine were refluxed for 50 hours at 40°C under the protection of argon in the solvent dichloromethane, and the concentration of bromine was controlled to be 10 g / 100 ml of dichloromethane, and the amount of dichloromethane added was 100 Milliliters per gram of perylene diimide, after the reaction is complete, the mixed solution of dichloromethane and liquid bromine is distilled off at normal pressure and recovered to generate dibromoperylene diimide with a yield of 90%. Add 1.2 g of dibromoperylene diimide and 20 ml of pyrrolidine into a 50 ml single-necked round bot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com