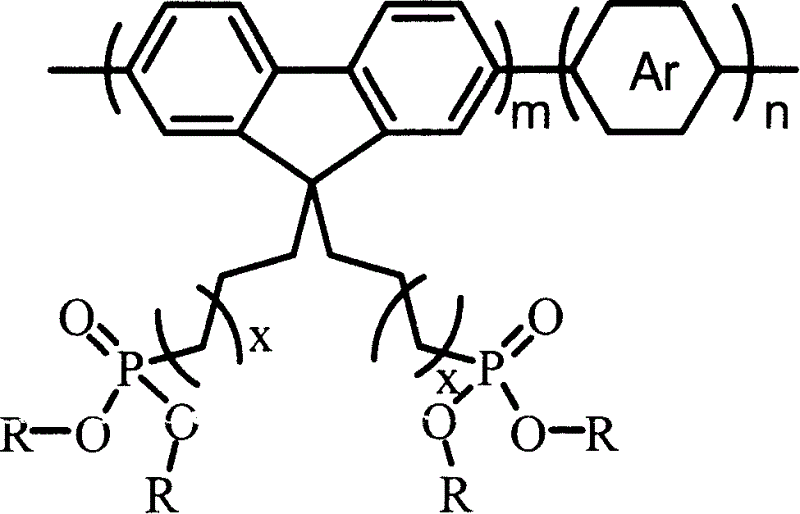
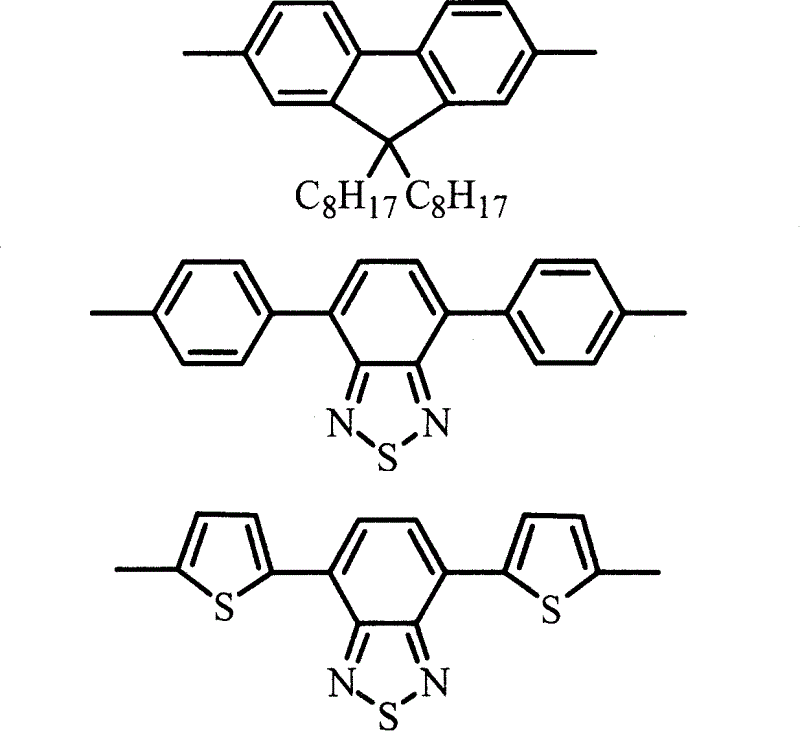
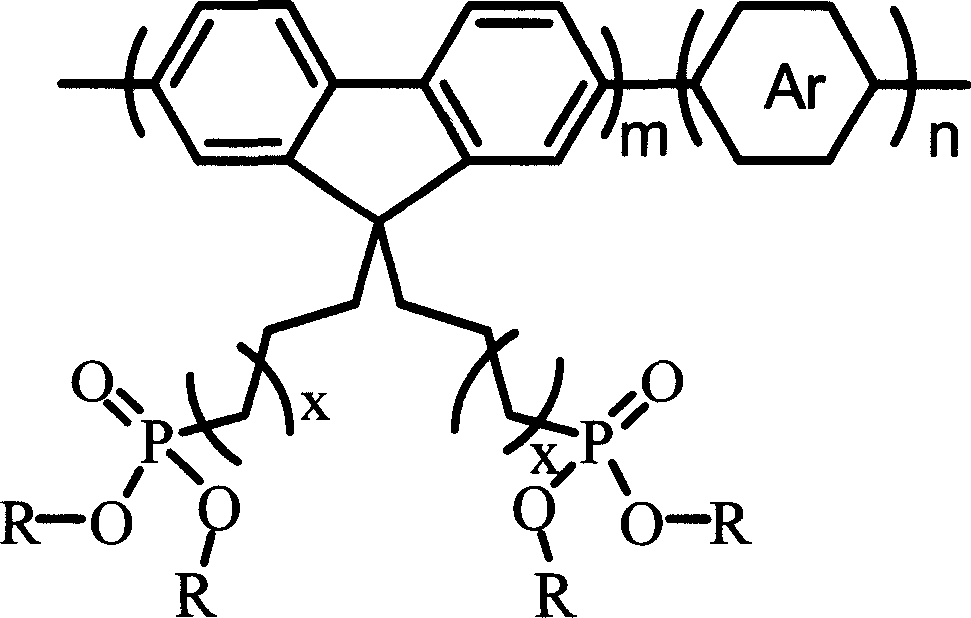
Alcohol soluble high molecular material in poly-fluorene group containing phosphate group and preparation method
A technology of phosphate groups and polymer materials, applied in the field of optoelectronic materials, which can solve the problems of few types of polymers, low molecular weight and solubility, poor color purity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: 2,7-dibromo-9, the synthesis of 9-(3'-bromopropyl) fluorene
[0018] at 10 5 Under Pa argon protection, add 5g of 2,7-dibromofluorene, 30mL of 1,3-dibromopropane, 0.1g of tetrabutylammonium bromide and 30mL of 50% sodium hydroxide in a 250mL three-necked reaction flask aqueous solution, stirred and reacted at 60°C for 10 hours. After the reaction, dilute with 100mL dichloromethane solution, then wash twice with 100mL water, 100mL 1M hydrochloric acid, 100mL water, 100mL brine, and wash the organic phase with MgSO 4 After drying, the solvent was removed to obtain a crude product, which was separated by column chromatography and petroleum ether was used as eluent to obtain 5.4 g of 2,7-dibromo-9,9-(3'-bromopropyl)fluorene, which was white Solid, yield 62%.
Embodiment 2
[0019] Embodiment 2: 2,7-dibromo-9, the synthesis of 9-(4'-bromobutyl) fluorene
[0020] at 10 5 Under the protection of Pa argon, 5g of 2,7-dibromofluorene, 10.9g of 4-(4'-bromobutoxy)toluene, 50mL of toluene, 0.1g of tetrabutyl bromide were successively added into a 250mL three-necked reaction flask Ammonium chloride and 30 mL of 50% aqueous sodium hydroxide solution were stirred and reacted at 60° C. for 10 hours. After the reaction, dilute with 100mL dichloromethane solution, then wash twice with 100mL water, 100mL 1M hydrochloric acid, 100mL water, 100mL brine, and wash the organic phase with MgSO 4 After drying, the solvent was removed to obtain a crude product, which was separated by column chromatography using petroleum ether as eluent to obtain 6.3 g of white solid with a yield of 65%. Reflux 6.3g of the above product in 4mL of 48% hydrobromic acid and 20mL of glacial acetic acid mixture at 110°C for 48 hours, pour it into water, extract with 100mL of dichloromethan...
Embodiment 3
[0021] Example 3: Synthesis of 2,7-dibromo-9,9-(6'-bromohexyl)fluorene
[0022] at 10 5 Under the protection of Pa argon, 5g of 2,7-dibromofluorene, 30mL of 1,6-dibromohexane, 0.1g of tetrabutylammonium bromide and 30mL of 50% hydroxide were successively added to a 250mL three-necked reaction flask Sodium aqueous solution, the reaction conditions and treatment method were the same as in Example 1 to obtain 8.3 g of 2,7-dibromo-9,9-(6'-bromohexyl)fluorene as a white solid with a yield of 81%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com