Method for preparing axcnic high pure oxymatrine
An oxymatrine, high-purity technology, applied in the directions of organic chemistry, bulk chemical production, etc., can solve the problems of complex process steps, low yield, high energy consumption, and achieve high extraction efficiency, large extraction concentration gradient, and high efficiency. The effect of dissipating phase change heat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
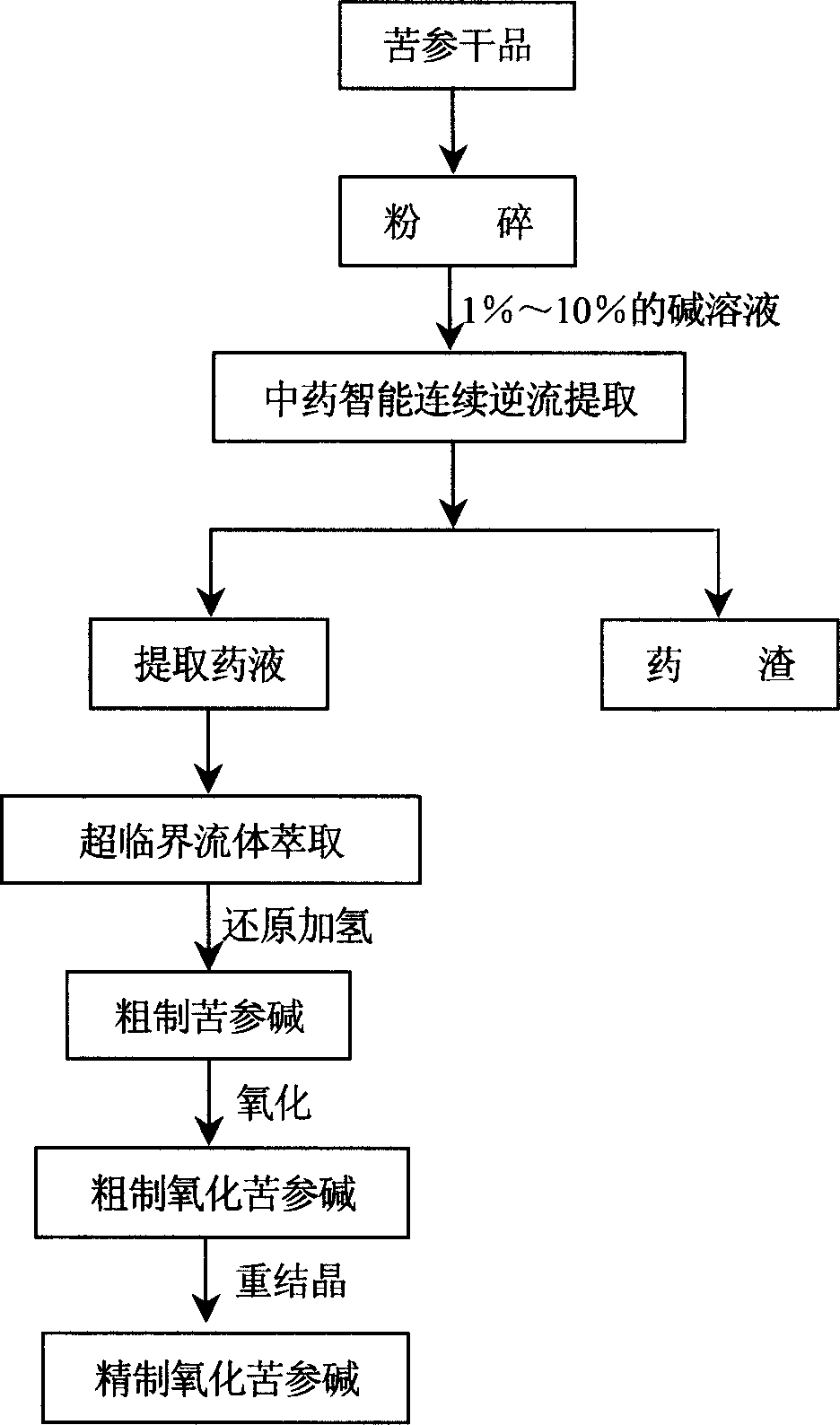
[0012] Specific embodiment 1: This embodiment prepares aseptic high-purity oxymatrine in this way: a. Select high-quality dried matrine as raw material, crush the raw material decoction pieces into coarse powder, pass through a 80-100 mesh sieve, and grind the coarse Put the powder into the intelligent continuous countercurrent extraction equipment of traditional Chinese medicine, add alkali solution to extract for 8-10 hours, and the amount of alkali solution is 50-60% of the material weight; 2 medium, under the conditions of pressure 20-30MPa and temperature 30-40°C, extract for 10-15min, extract twice and then spray dry to obtain extraction powder; c. Put the extraction powder into the reaction kettle and use appropriate Dissolve the powder in EtOH, add the catalyst, seal the reaction vessel, and feed in N 2 To evacuate the air, and then introduce H at a pressure of 2 to 5 atmospheres 2 React for 2 to 3 hours, the reaction is over, and then centrifuged (the separated catal...
specific Embodiment approach 2
[0013] Specific embodiment two: this embodiment prepares oxymatrine according to the technical process in Fig. 1, and the specific technical process is as follows: a, select 500Kg of Sophora japonica produced in Northeast China, grind it into coarse powder, pass through an 80-mesh sieve, and pack the coarse powder into traditional Chinese medicine Intelligent continuous countercurrent extraction equipment, add sodium hydroxide solution with a weight concentration of 5% (the amount is 60% of the material weight) to extract for 8 to 10 hours; b, add the extract to the supercritical extraction kettle, and feed CO 2 Medium, the pressure is controlled at 20-30MPa, the temperature is controlled at 30-40°C, and the time is 10-15min. Continuously extract twice, and then spray dry to obtain the extracted powder; c. Put the extracted powder into the reaction kettle, and use an appropriate amount of Dissolved EtOH, added Ni as a catalyst (the weight ratio of the catalyst to the extraction...
specific Embodiment approach 3
[0014] Specific embodiment three: This embodiment prepares oxymatrine according to the process flow shown in Figure 1. The specific process is as follows: a. Select 500Kg of Sophora flavescens produced in Northeast China, grind it into coarse powder, pass through a 100-mesh sieve, and pack the coarse powder into the traditional Chinese medicine intelligence Continuous countercurrent extraction equipment, adding an alkali solution with a weight concentration of 8% (the amount of alkali solution is 50% of the weight of the material) to extract for 8 to 10 hours; b. Add the extract to the supercritical extraction kettle, and feed CO 2 Medium, the pressure is controlled at 20-30MPa, the temperature is controlled at 30-40°C, and the time is 10-15min. Continuously extract twice, and then spray dry to obtain the extracted powder; c. Put the extracted powder into the reaction kettle, and use an appropriate amount of Dissolved EtOH, added Ni as a catalyst (the weight ratio of the cataly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com