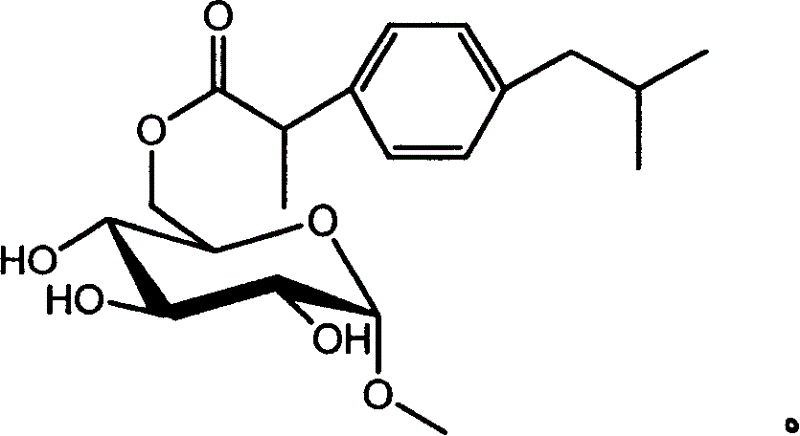
Ibuprofen sugar derivative and its preparing process and use
A technology of sugar derivatives and glucosides, which is applied in the field of 6--ibuprofen and its preparation, can solve the problems of low bioavailability, increased gastrointestinal side effects, and clinical application limitations, and achieve the effect of simplifying the operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] In a 50ml ground-mouth Erlenmeyer flask, add 20ml methyl ethyl ketone, 0.2g ibuprofen (about 1mmol) and α-methyl-D-glucoside (about 1mmol) and 2.0g activated molecular sieves (3.0 Å), at 50°C Shake for a certain period of time, and then add 0.2 g of commercial lipase Novozym 435 after the reaction substrate is fully dissolved. By thin-plate chromatography TLC (CHCl 3 :CH 3 OH:H 2 O (65:15:2)) to detect the reaction process. After reacting for 96hr, the reaction liquid was cooled, 40ml of dichloromethane was added, shaken at room temperature for 20min, and the immobilized lipase was floated out, then filtered, and rotary evaporated at 40°C, and the residue was cleaned with a silica gel column (ethyl acetate:methanol, 10: 1 v / v) to obtain 6-(α-methyl-D-glucoside)-(±) ibuprofen. The melting point was determined to be 52-55°C with a melting point analyzer (uncorrected).
[0024] The mass-to-nucleus ratio m / z of the compound was verified to be 382 by mass spectrometry. ...
Embodiment 2
[0029] In a 50ml ground-neck Erlenmeyer flask, add 20ml of acetonitrile, 0.2g of ibuprofen (about 1mmol) and α-methyl-D-glucoside (about 1mmol) and 2.0g of activated molecular sieves (3.0 Å), at 50°C Shake for a certain period of time, and then add 0.2 g of commercial lipase Novozym 435 after the reaction substrate is fully dissolved. By thin-plate chromatography TLC (CHCl 3 :CH 3 OH:H 2 O (65:15:2)) to detect the reaction process. After reacting for 96hr, the reaction liquid was cooled, 40ml of dichloromethane was added, shaken at room temperature for 20min, and the immobilized lipase was floated out, then filtered, and rotary evaporated at 40°C, and the residue was cleaned with a silica gel column (ethyl acetate:methanol, 10: 1 v / v) to obtain 6-(α-methyl-D-glucoside)-(±) ibuprofen. The yield of the target product was 30.3%.
Embodiment 3
[0031] In a 50ml ground-neck Erlenmeyer flask, add 20ml of acetonitrile, 0.8g of ibuprofen (about 4mmol) and 0.1g of α-methyl-D-glucoside (about 0.5mmol), shake at 50°C for a certain period of time, wait After the reaction substrate was fully dissolved, 0.2 g of commercial lipase Novozym 435 was added. By thin-plate chromatography TLC (CHCl 3 :CH 3 OH:H 2O (65:15:2)) to detect the reaction process. After reacting for 96hr, the reaction liquid was cooled, 40ml of dichloromethane was added, shaken at room temperature for 20min, and the immobilized lipase was floated out, then filtered, and rotary evaporated at 40°C. 1 v / v) to obtain 6-(α-methyl-D-glucoside)-(±) ibuprofen. The target product yield was 78.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com