Heavy metal adsorbent composition
A heavy metal and adsorbent technology, applied in the direction of adding compounds to stimulate growth, microorganisms, microorganisms, etc., can solve the problems of limited use and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 (selection and identification of heavy metal adsorbing bacteria)
[0050] (1) Selection of heavy metal adsorbing bacteria
[0051] The soil was suspended in normal saline and allowed to stand, and the supernatant was cultured in BrainHeart Infusion Agar medium containing 1mM heavy metals, and the colonies that appeared were selected after 1 day.
[0052] (2) Identification of the obtained strain
[0053] a.method
[0054] The first phase of the bacteria test was to observe the morphology of the cells, Gram staining, presence or absence of spores, and presence or absence of flagellar movement with an optical microscope U-LH1000 (Olympus, Japan). Colony morphology was observed on a medium containing Brain Heart InfusionAgar (Becton Dickinson, NJ, USA) and agar (B.H.I agar). Catalase reaction, oxidase reaction, acid / gas generation from glucose, glucose oxidation / fermentation (O / F) tests were performed.
[0055] The second stage of the test for bacteria is ...
Embodiment 2
[0067] After culturing KRI-02, KRI-03, and KRI-04 in Brain Heart Infusin Medium (Difco), they were washed with water, and 0.5N hydrochloric acid was added in a volume equivalent to 5 times the wet weight of the bacteria to suspend them. Then, the bacteria added with hydrochloric acid were shaken at 37° C. for 2 hours. In addition, the method of Brierley et al. (USP 4,992,179) was studied comparatively. That is, 3% sodium hydroxide in a volume equivalent to 5 times the wet weight will be added, and the bacteria will be shaken at 50°C or 100°C for 10 minutes. After shaking, each strain was thoroughly washed with water and freeze-dried. The results are shown in Table 5. Compared with the case of washing with water (untreated), the acid treatment reduced the weight by 20%, while the sodium hydroxide treatment reduced the weight by more than 50%, especially when treated at 100°C, Weight reduction over 60%.
[0068] Approach
Embodiment 3
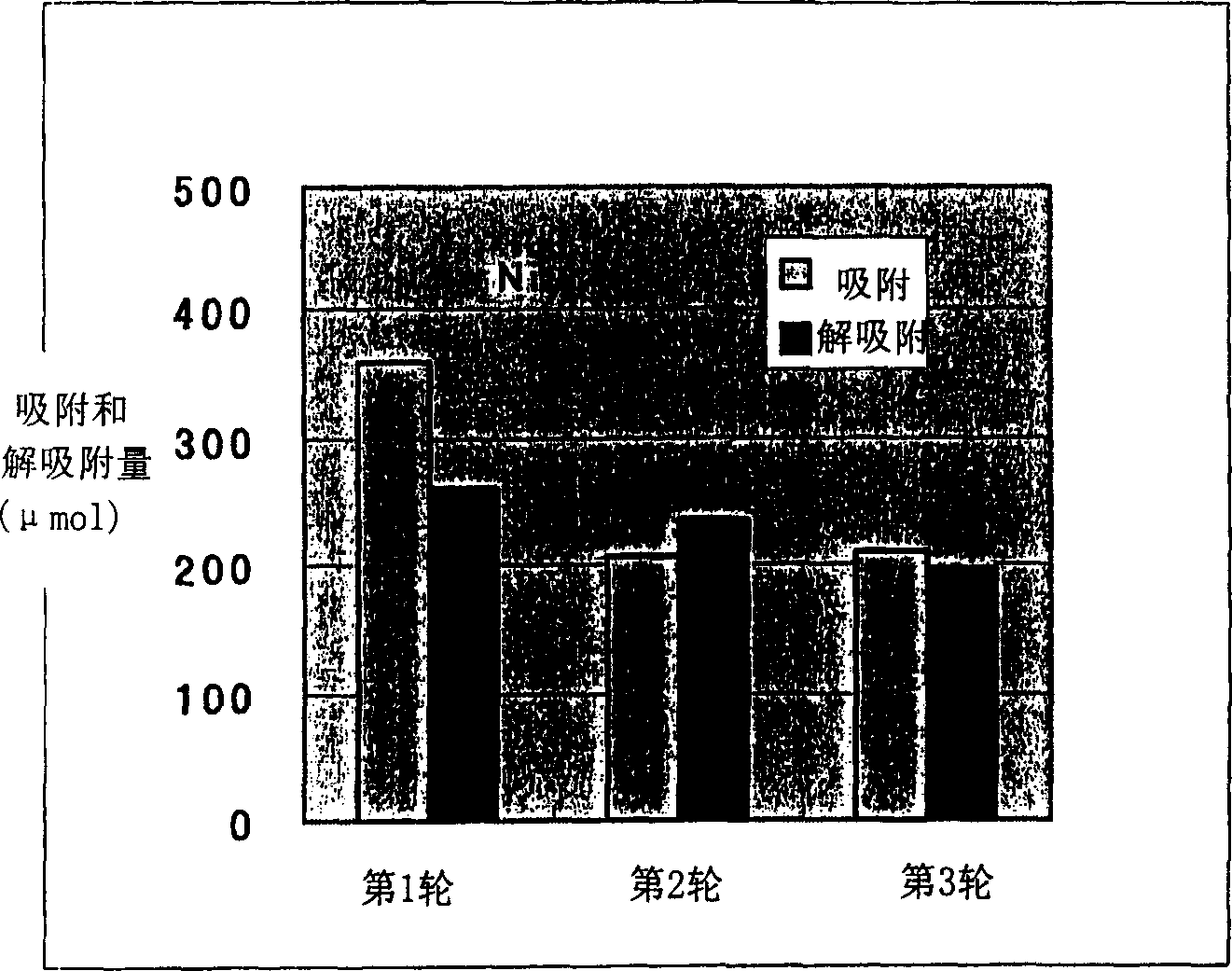
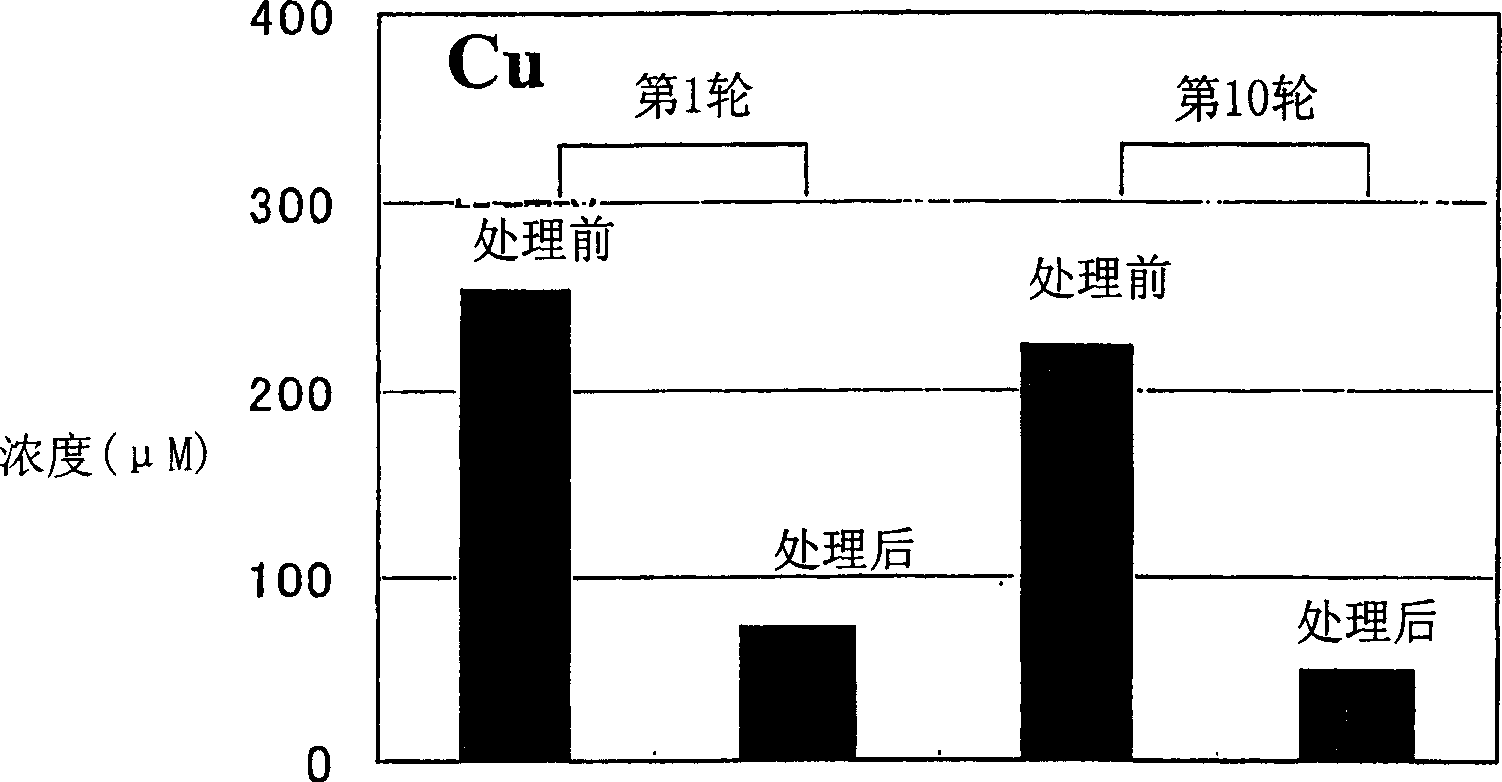
[0069] Embodiment 3 (measurement of metal adsorption amount)
[0070] The freeze-dried bacterial powder was dispersed in a buffer solution (Tris: 100 mM) to prepare a 60 mg / ml suspension. Add 20 microliters of bacterial suspension to 2.4 mM heavy metal aqueous solution (CdCl) prepared by Tris (10 mM). 2 , CuSO 4 , ZnCl 2 , NiCl 2 ) in 1 ml. Centrifuge after the reaction, and measure the heavy metal concentration in the separated supernatant with an atomic absorption photometer.
[0071] The results are shown in Tables 6-9. Compared with washing with water, the adsorption capacity of KRI-02, KRI-03, and KRI-04 after acid treatment increased to cadmium and copper. Although the cadmium adsorption of KRI-02, KRI-03, and KRI-04 treated with sodium hydroxide also increased compared with water washing, the acid treatment increased more than that of sodium hydroxide. Acid-treated KRI-02, KRI-03, KRI-04 showed increased adsorption of Zn and Ni than water-washed, but NaOH treatme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com