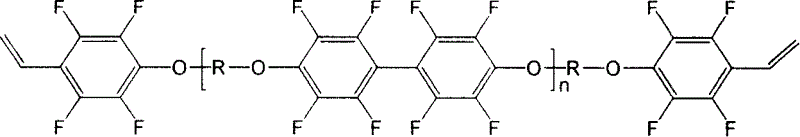
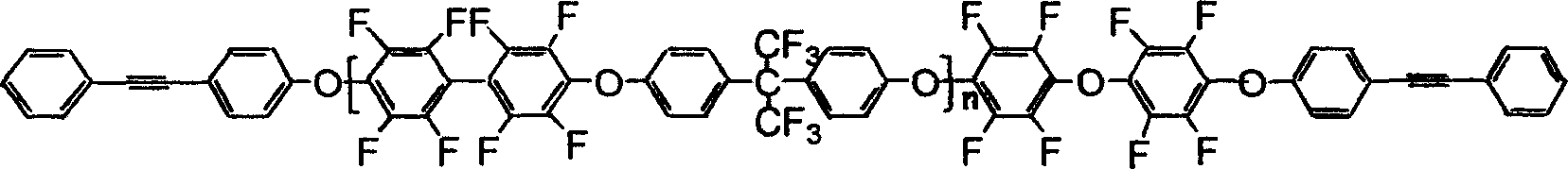
Preparation of photosensitive fluorine-containing poly(aryl ether) materials and use thereof
A technology of polyarylether and raw materials, which is applied in the field of polymer materials and its preparation, can solve problems affecting material properties, poor thermal expansion coefficient, high crosslinking temperature, etc., and achieve low dielectric constant, high thermal stability, and low light loss Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 5.08 g (20 mmol) of m-trifluoromethylbenzene pendant hydroquinone (3F-PH) and 3.312 g of anhydrous potassium carbonate into a three-necked flask equipped with a stirring bar and a thermometer, and protect it from light and nitrogen. Add 49ml of N,N-dimethylacetamide (solid content 10%), stir the toluene until it is completely dissolved, then raise the temperature to 120°C with water for 2 hours, and the bisphenol will form a salt. After cooling to room temperature, add 0.388g (2mmol) of pentafluorostyrene, rapidly raise the temperature to 114°C for 20 minutes, and cool to room temperature; dissolve 6.346g (19mmol) of decafluorobiphenyl in 61ml of N,N-dimethylacetamide Add the reaction system, rapidly raise the temperature to 114°C and react for 20 minutes to obtain a linear polymer.
Embodiment 2
[0032] Add 5.08 g (20 mmol) of m-trifluoromethylbenzene pendant hydroquinone (3F-PH) and 3.312 g of anhydrous potassium carbonate into a three-necked flask equipped with a stirring bar and a thermometer, and protect it from light and nitrogen. Add 31ml of N,N-dimethylacetamide (15% solid content), stir the toluene until it is completely dissolved, then raise the temperature to 120°C with water for 2 hours, and the bisphenol will form a salt. After cooling to room temperature, add 0.388g (2mmol) of pentafluorostyrene, rapidly raise the temperature to 114°C for 20 minutes, and cool to room temperature; dissolve 6.346g (19mmol) of decafluorobiphenyl in 38ml of N,N-dimethylacetamide Add the reaction system, rapidly raise the temperature to 114°C and react for 20 minutes to obtain a linear polymer.
Embodiment 3
[0034] Add 5.08 g (20 mmol) of m-trifluoromethylbenzene pendant hydroquinone (3F-PH) and 3.312 g of anhydrous potassium carbonate into a three-necked flask equipped with a stirring bar and a thermometer, and protect it from light and nitrogen. Add 10ml of N,N-dimethylacetamide (solid content 35%), stir the toluene until it is completely dissolved, then raise the temperature to 120°C with water for 2 hours, and the bisphenol will form a salt. After cooling to room temperature, add 0.388g (2mmol) of pentafluorostyrene, rapidly raise the temperature to 114°C for 20 minutes, and cool to room temperature; dissolve 6.346g (19mmol) of decafluorobiphenyl in 13ml of N,N-dimethylacetamide Add the reaction system, rapidly raise the temperature to 114°C and react for 20 minutes to obtain a linear polymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com