Co-Fe-B amorphous alloy catalyst, its preparation method and application
A technology of amorphous alloy and catalyst, which is applied in the field of Co-Fe-B amorphous alloy catalyst and its preparation, can solve the problems of insufficient catalytic activity and selectivity, and achieve excellent catalytic activity and selectivity, excellent catalytic performance and The effect of activity selectivity and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
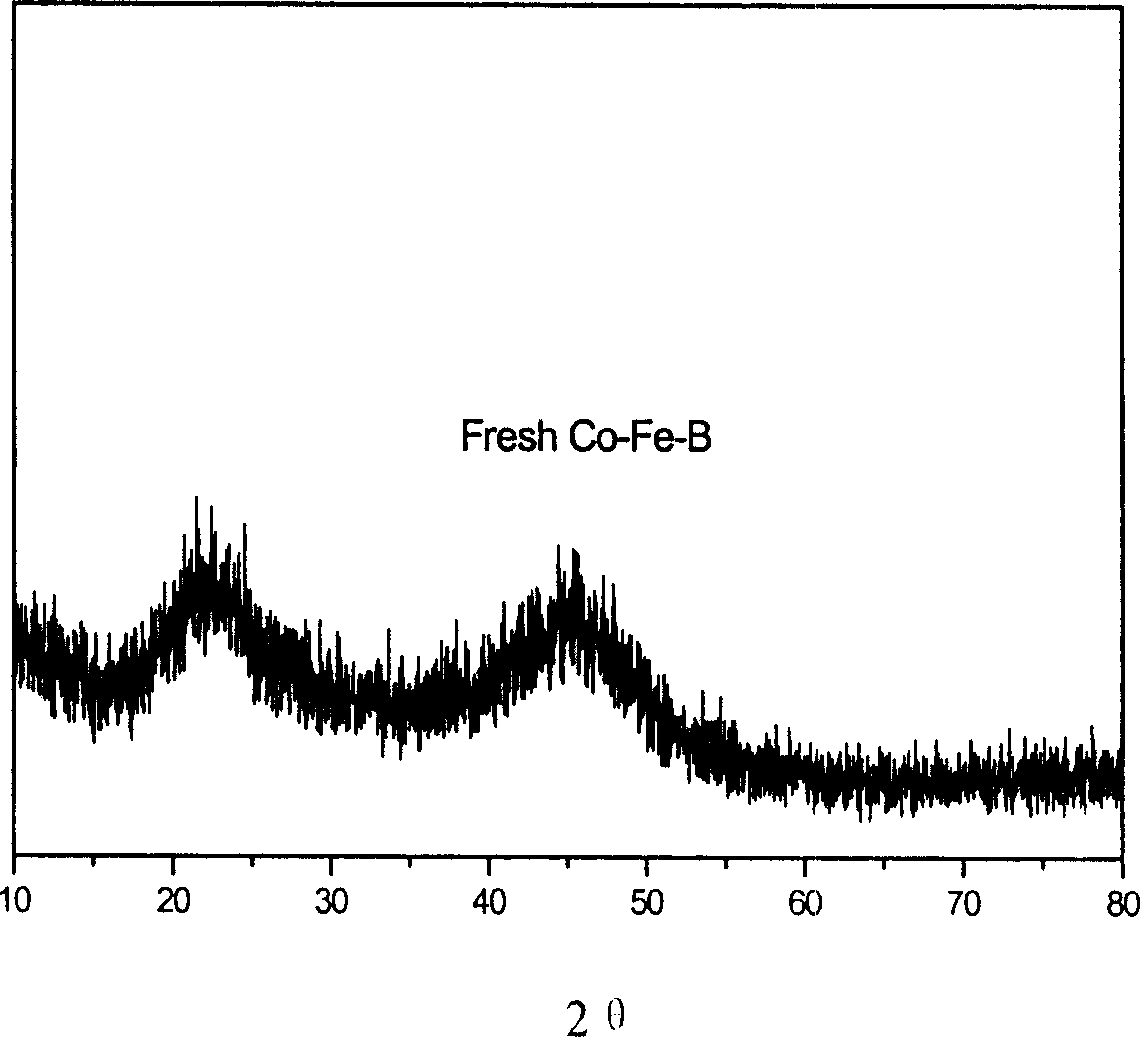
[0021] KBH 4 A mixed solution with a concentration of 2mol / L and a NaOH concentration of 0.2mol / L was added dropwise to CoCl 2 and FeCl 3 mixed solution of Fe 3+ with Co 2+ The molar ratio of 1:5, control KBH 4 and Co 2+ and Fe 2+ The molar ratio was 5:1, and the reaction was carried out for 30 minutes to ensure the complete reduction of metal ions. The reaction temperature was 10°C, and then the prepared particles were washed with deionized water until neutral, and then washed with absolute ethanol to remove water, and the Co- Fe-B amorphous alloy catalyst, stored in absolute ethanol. The molar percentage of each component of the catalyst thus obtained is analyzed by ICP: Co: 26.9%, Fe: 5.2%, B: 67.9%, totaling 100%, and the specific surface area measured by the BET method is 55.3m 2 / g. X-ray diffraction pattern see figure 1 .
Embodiment 2~10
[0023] Using the same method as in Example 1, wherein the process parameters are shown in Table 2. Fe 3+ with Co 2+ The mol ratio is shown in Table 1:
[0024] Example
[0025] implement
Embodiment 11~16
[0027] The catalyzer of embodiment 2~10 is used for acetonitrile liquid phase selective hydrogenation to prepare ethylamine reaction, and the initial pressure of hydrogenation reaction is PH 2 =3.0MPa, the reaction temperature is controlled at 110°C, and the reaction is carried out in a 0.5L autoclave. Add 1.5g of the prepared catalyst, 30ml of acetonitrile, and 90ml of ethanol, and react until the conversion rate of acetonitrile is 100%. The results are shown in Table 3:
[0028] catalyst
[0029] It can be seen from Table 3 that the Co-B amorphous alloy catalyst doped with a certain amount of Fe can greatly increase the initial hydrogen absorption rate in the liquid phase hydrogenation reaction of acetonitrile. And the Co-Fe-B catalyst doped with 20wt% Fe has the best catalytic activity, reaching 987.4mmol / h·gCo, and the reaction rate increases by 183.3% compared with the Co-B catalyst without Fe doping.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com