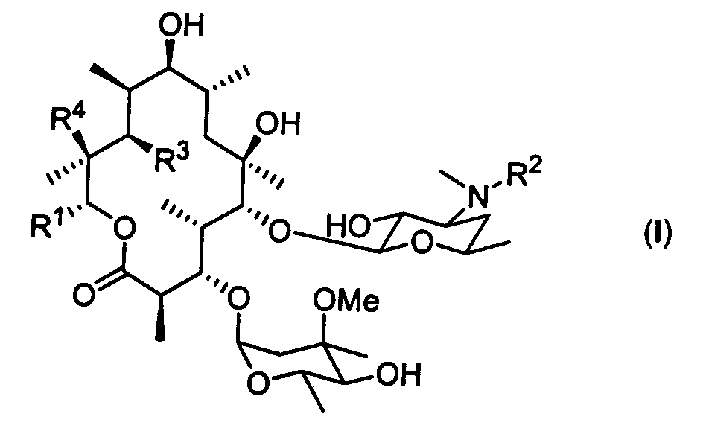
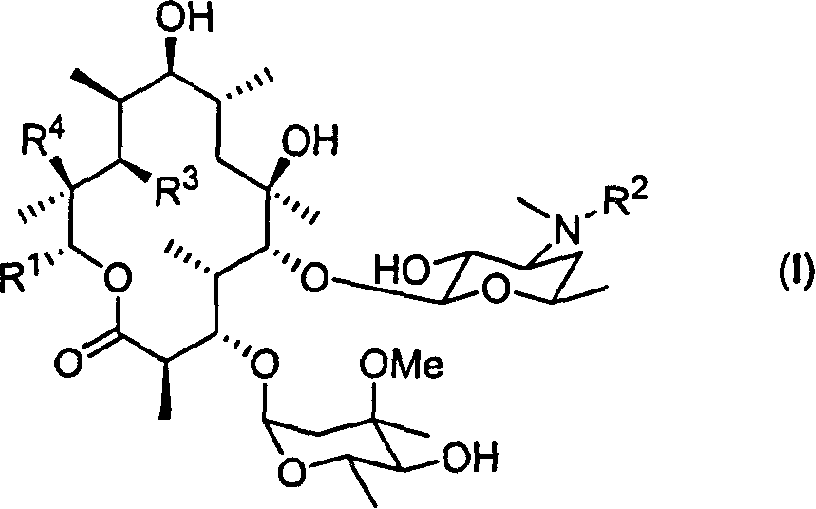
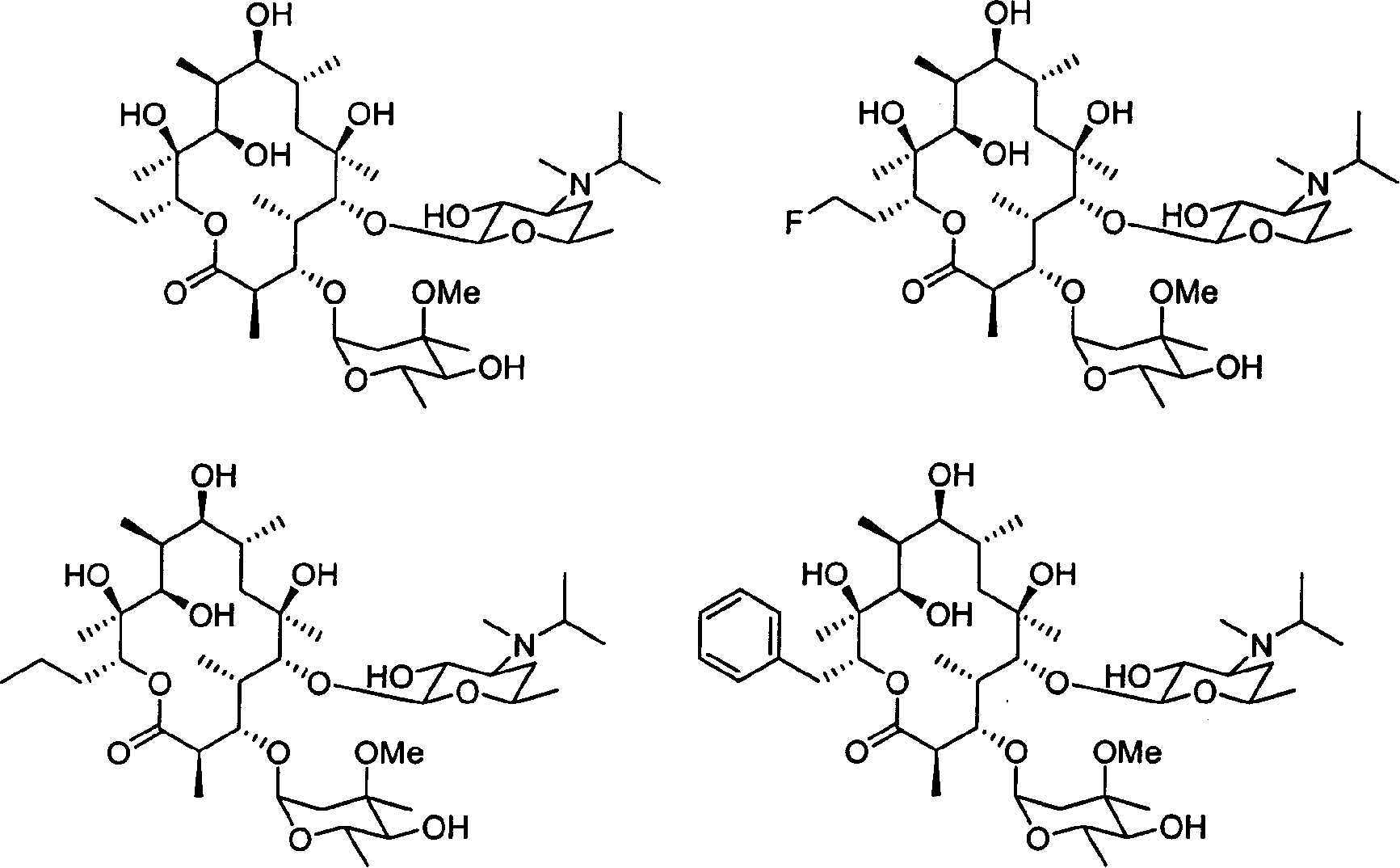
Motilide compounds
A compound and prodrug technology, applied in the field of prokinetic preparations, can solve problems such as the decline in the efficacy of kinetolactones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] This example describes the manufacture of 15-methyl-6-deoxyerythromycin B (also known as 13-propyl-6-dEB or 15-methyl-6-dEB), which was used to synthesize the Intermediates for specific compounds. (Other erythromycins are similarly named; for example, erythromycin with a methyl group replaced by a fluorine atom at position 15 is called 15-fluoro-6-dEB).
[0093] 15-Methyl-6-deoxyerythromycin B
[0094] Thaw the CH999 / pJRJ2 (Streptomyces coelicolor, which contains PKS in which the ketone synthase domain of module 1 is inactivated by mutation) working cell bank (working cell bank) in the 1 ml vial, and the contents of the vial Add to 50 mL of Medium 1 in a 250 mL stoppered flask.
[0095] Medium 1 contains 45 g / L cornstarch, 10 g / L corn steep liquor, 10 g / L dry, inactivated S. cerevisiae, and 1 g / L CaCO 3 . The solution was sterilized in an autoclave at 121°C for 90 minutes. After sterilization, add 1 mL / L sterile-filtered 50 mg / mL thiostrepton in 100% DMSO and 1 m...
Embodiment 2
[0103] This example describes the conversion of 15-methyl-6-deoxyerythromycin B into 15-methylerythromycin A (formula II, R 1 =-CH 2 CH 2 CH 3 ; 3 =R 4 =OH). Transformation techniques are also found in Carreras et al., J. Biotechnology, 92, pp. 217-228 (2002), the entire contents of which are hereby incorporated by reference.
[0104] Thaw working cell bank K39-14V (an eryA mutant of S. rubrum that cannot produce 6-dEB) in a 1 ml vial and add the contents of the vial to 50 ml of a 250 ml stoppered flask Medium 2.
[0105] Medium 2 consisted of 16 g / L cornstarch, 10 g / L corn dextrin, 15 g / L soy meal, 4 g / L CaCO 3 , 5 g / L corn steep liquor, 6 g / L soybean oil, 2.5 g / L NaCl, and 1 g / L (NH 4 ) 2 SO 4 . The solution was autoclaved at 121°C for 60 minutes, and 1 ml / L of autoclaved 100% Foam B Silicone Emulsion was added just before use.
[0106]Place the flask containing thawed cells and Medium 2 in an incubator / mixer at 34±1°C and 175±25 RPM for 48±10 hours. Then, 50 ml...
Embodiment 3
[0115] This example describes a conventional method for preparing (9S)-9-dihydroerythromycin (Formula II), see Scheme 1 for details.
[0116] A solution of erythromycin (0.36 mmol) in 1:3 ethanol / ether (20 mL) was cooled to -15°C and treated with sodium borohydride (0.9 mmol). The reaction was allowed to warm slowly to ambient temperature over 4 hours. Excess borohydride was removed by adding pH 6 phosphate buffer, and 10 mL of triethanolamine was added. After 1 h, the mixture was extracted with ethyl acetate and washed with MgSO 4 Dry, filter, and concentrate to dryness under reduced pressure. The product was purified by silica gel chromatography using 1:1 acetone-hexane and 1% triethylamine. The following compounds were prepared using this procedure:
[0117] (9S)-9-dihydroerythromycin A;
[0118] (9S)-9-dihydro-15-methylerythromycin A; and
[0119] (9S)-9-dihydro-15-fluoroerythromycin A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com