Compound extracted from eucalyptus plants and use thereof
A technology of compounds and uses, applied in the field of application in the treatment of tumors, can solve problems such as insufficient research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] New compound extracted from eucalyptus
[0068] A. Extracted from blue eucalyptus fruit
[0069] (A1) Extraction process
[0070] Extract as follows:
[0071] 1. Take the pulverized medicinal material (the fruit of Eucalyptus blueus, commonly known as a bell), extract it with 95% ethanol at room temperature for two weeks, and reclaim the ethanol solution to obtain the ethanol extract.
[0072] 2. Take the ethanol extract, extract it with petroleum ether first, then extract it with ethyl acetate, and recover the ethyl acetate solvent to obtain the ethyl acetate part.
[0073] 3. Take an appropriate amount of ethyl acetate and separate it on a silica gel column. The elution gradient is petroleum ether: ethyl acetate = 10:1, petroleum ether: ethyl acetate = 5:1, petroleum ether: ethyl acetate = 3:1, petroleum ether: ethyl acetate = 2:1
[0074] 4. Collect the fraction eluted with petroleum ether: ethyl acetate = 2:1, and concentrate to obtain a brown-red viscous substa...
Embodiment 2
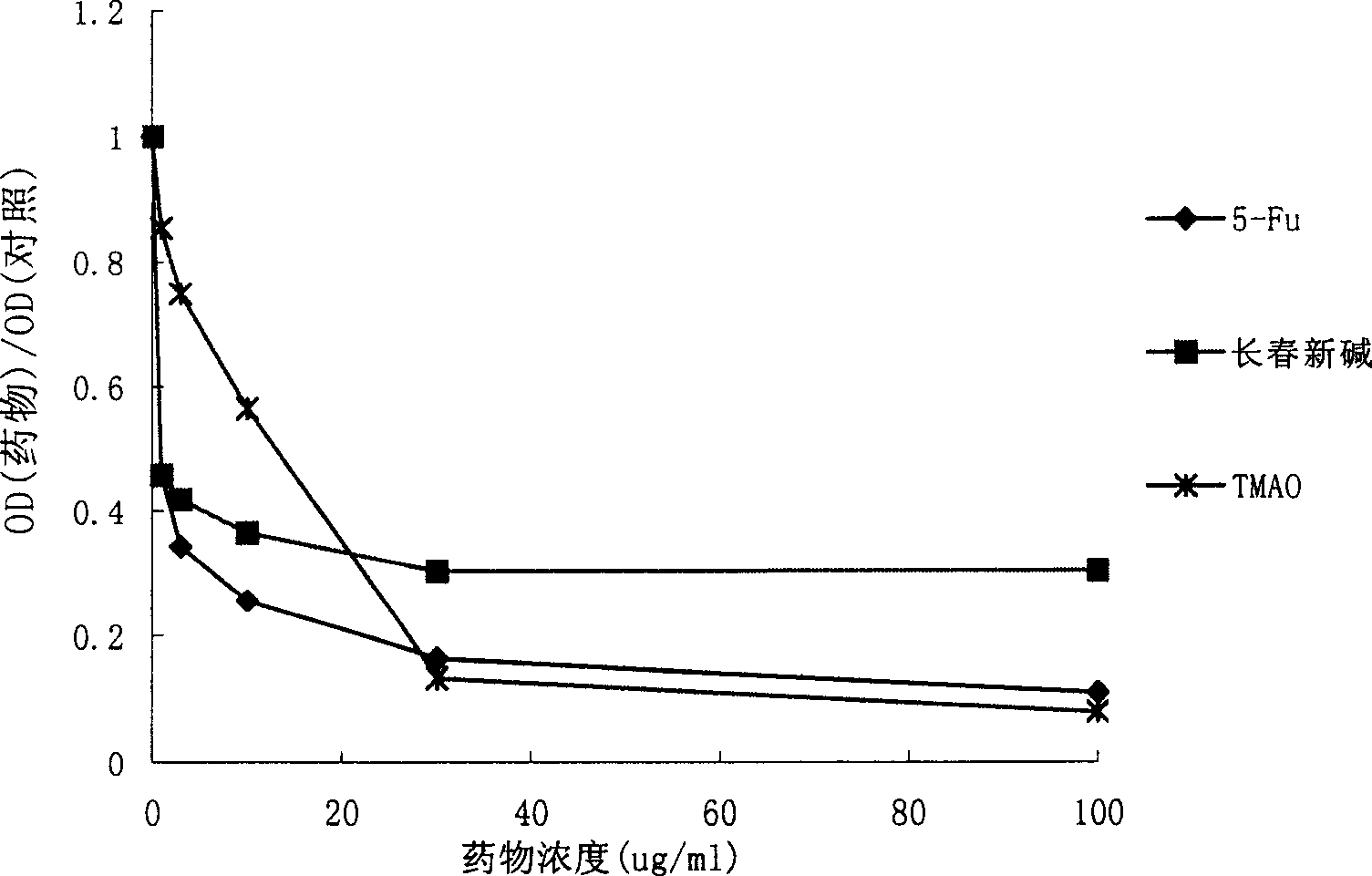
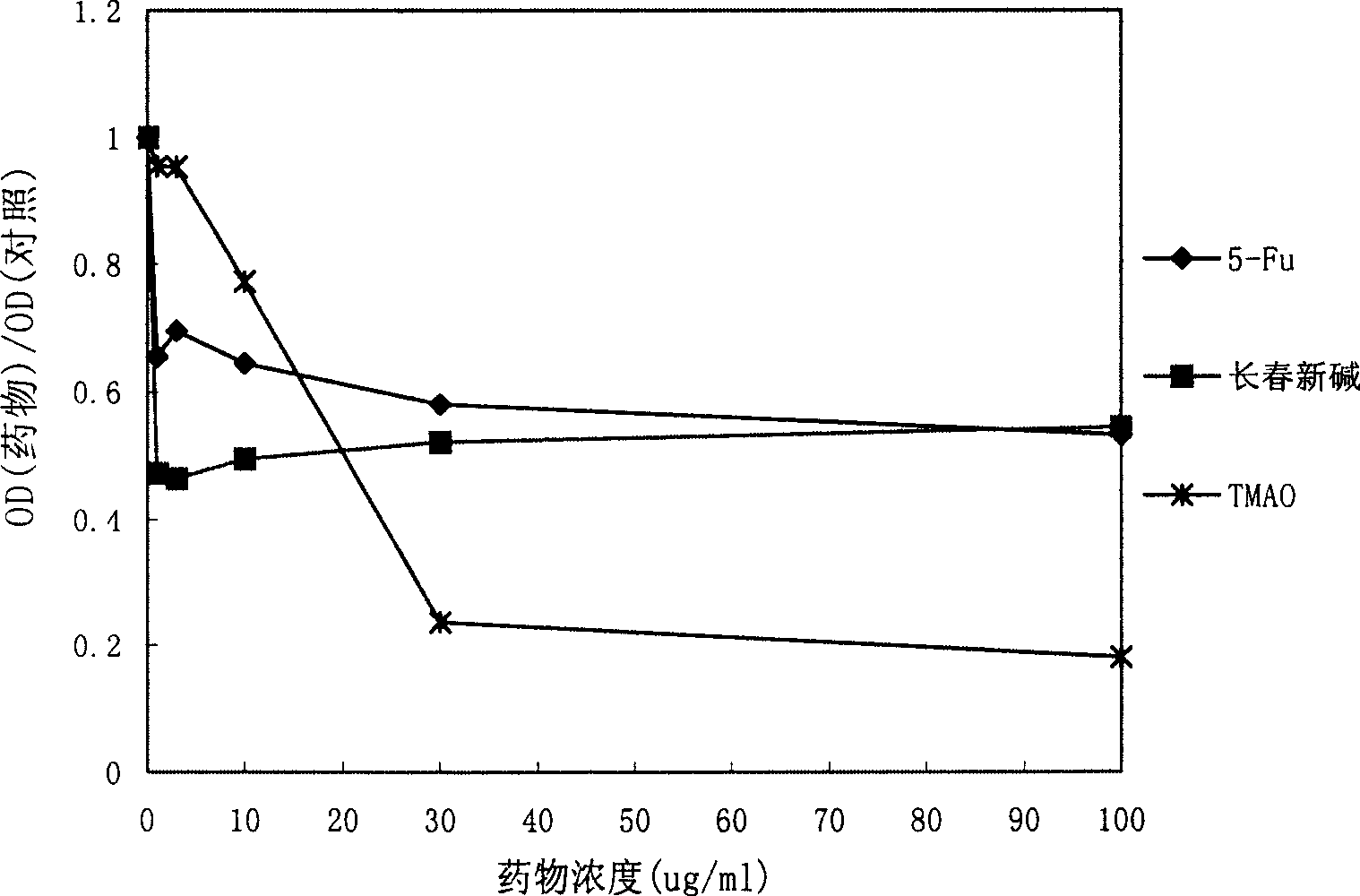
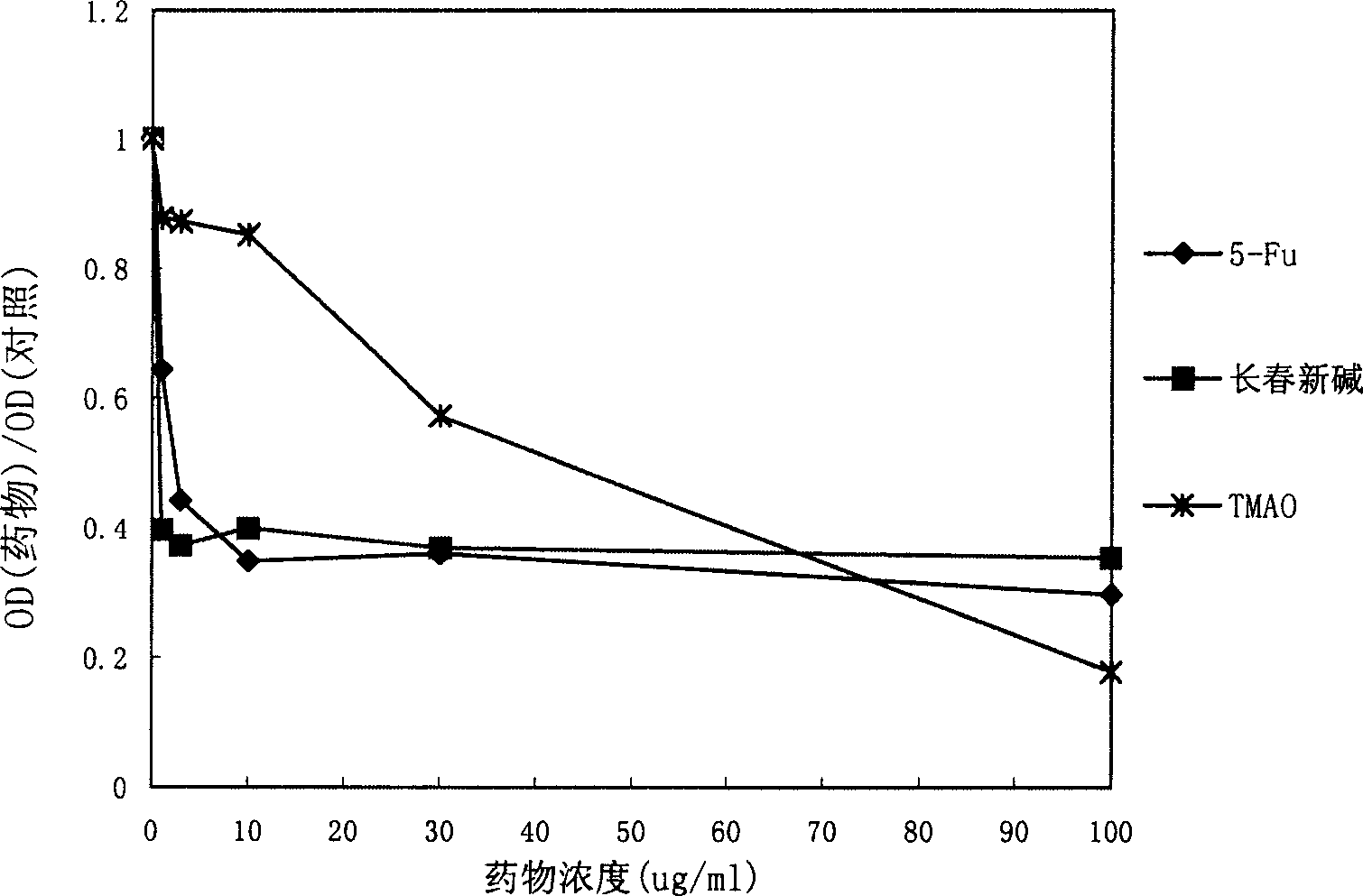
[0098] Study on anti-tumor effect in vitro
[0099] Huh-7 human liver cancer cell line, Du-145 human prostate cancer cell line, K562 human leukemia cell line, AGS human gastric cancer cell line, Eca-109 human esophageal cancer cell line, 786-0 human kidney cancer cell line, A549 human Lung cancer cell lines and CS-174-T human colon cancer cell lines were used for drug anti-tumor experiments in vitro. Cells were cultured in culture flasks until the number of cells reached 1×10 7 indivual. After the cells were digested, the cells were diluted to 3×10 with DMEM medium (GIBCO Company) 4 / ml. Take a 96-well plate, add 200ul diluted cell solution to each well, and store at 37°C in 5% CO 2 Incubation in the incubator. After 24 hours, discard the original cell culture medium, add 200ul of solutions containing different concentrations of various test drugs prepared with DMEM culture medium to the corresponding wells, and then continue to place the cell culture plate at 37°C in 5% ...
Embodiment 3
[0104] Study on anti-tumor effect in vivo
[0105] Huh-7 human liver cancer cells were routinely cultured. Take cells with good activity in the proliferative phase, with a volume of 0.25ml and a concentration of 1×10 7 The dose of cells / mouse was inoculated subcutaneously on the outer right thigh of nude mice (purchased from Shanghai Experimental Animal Center, Chinese Academy of Sciences). The next day after inoculation, the mice were randomly divided into 2 groups, 10 in each group. After raising for 30 days, give each mouse of the test drug group intraperitoneal injection of medicine (100mg / kg) solution 0.5ml every day; each mouse of the negative control group is given equal volume of normal saline every day; each group of mice is given 1 dose every day times, continuous administration for 10 days. After 24 hours of drug withdrawal, the mice in each group were sacrificed by bleeding, autopsy was performed, the tumor mass was taken out and weighed, and the tumor inhibitio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com