Tetraphenyl porphyrin derivative and its application in organic electroluminescent device
A technology of tetraphenylporphyrin and its derivatives, which is applied in the application field of tetraphenylporphyrin derivatives and their luminescent materials in organic electroluminescent devices, and can solve the problem of not having carrier transport performance, external quantum Low efficiency, poor stability and other issues, to achieve the effect of being conducive to mass industrial production of devices, high luminous efficiency and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] Example 1: Synthesis of Compound 1-1-01
[0036] Using 150 milliliters of N,N-dimethylformamide as a solvent, add 3.0 grams of sodium hydride, 10 grams of carbazole and 20.0 grams of 1,4-dibromobutane, and react under reflux for 6 hours. Cooling, add 50 milliliters of methanol and fully stir and filter, the collected filtrate is concentrated and evaporated to dryness, and the crude product uses cyclohexane as the eluent aluminum oxide as the stationary phase column chromatography to obtain the product N-(4-bromobutyl ) carbazole 8.2 grams. Yield 45.4%.
[0037] 10.0 grams of compound 3-methoxy-4-hydroxybenzaldehyde, 4.4 grams of pyrrole and 600 milliliters of N, N-dimethylformamide were heated to reflux for 2 hours, cooled and stilled, 200 milliliters of distilled water was added, filtered, and the crude product was used Dichloromethane was used as the eluent and aluminum oxide was used as the stationary phase column chromatography to obtain 3.5 grams of the product t...
example 2
[0042] Example 2: Synthesis of Compound 1-1-02
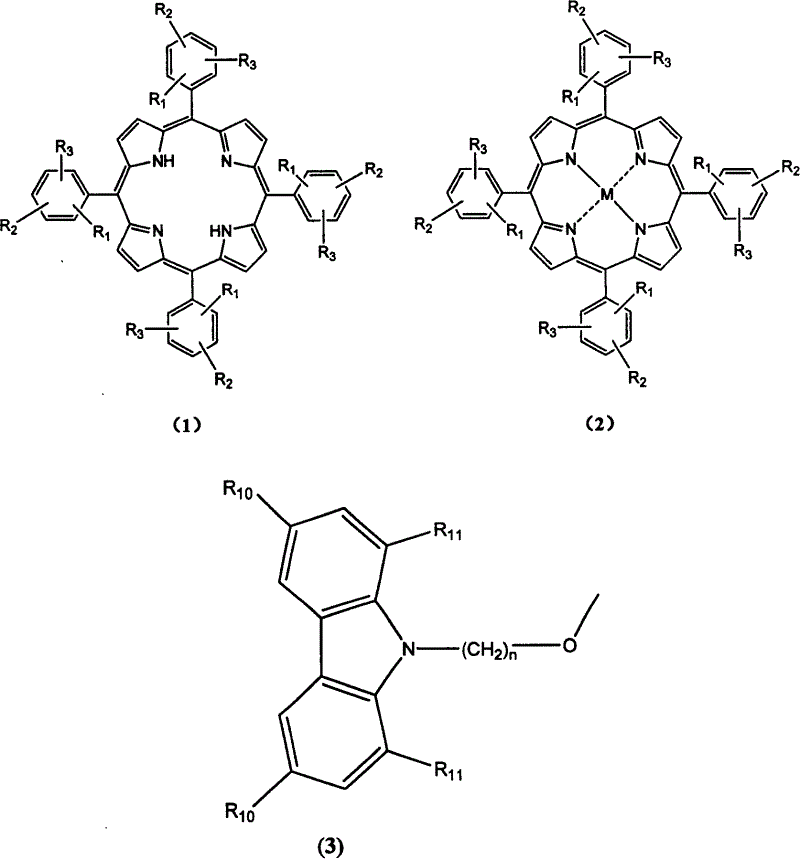
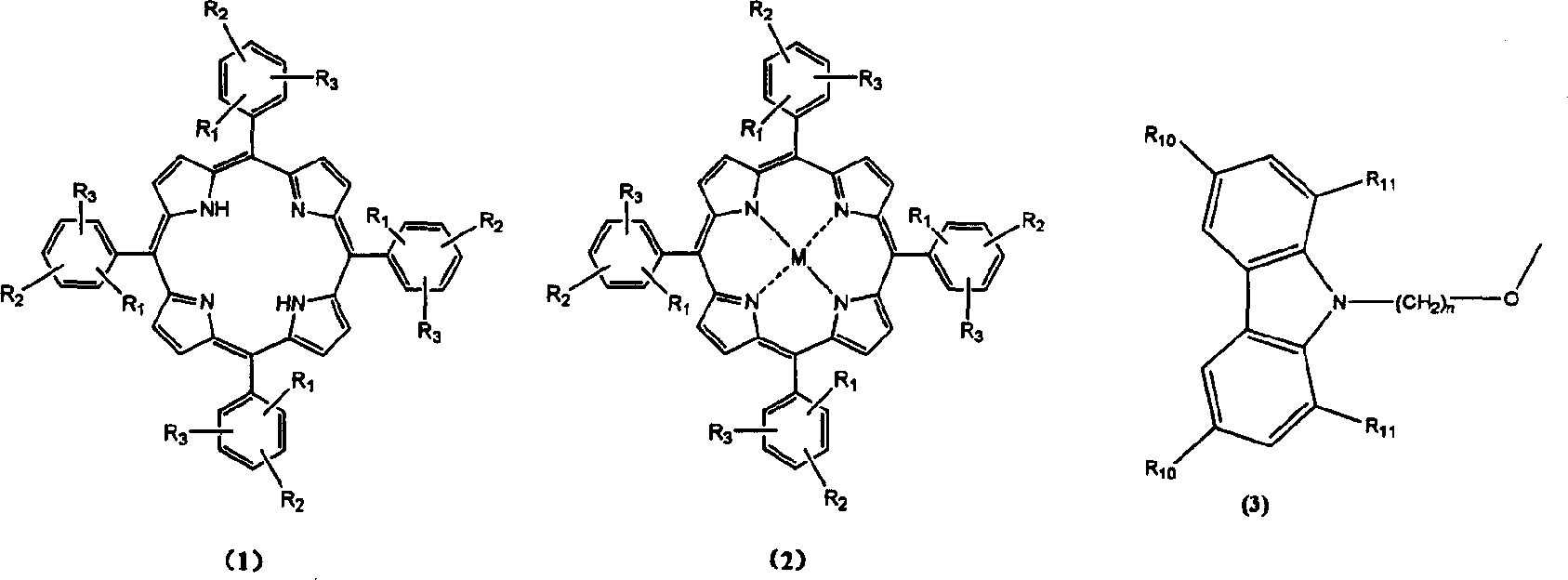
[0043] The synthesis of compound 1-1-02 was the same as in Example 1. Just use 1,5-dibromopentane instead of 1,4-dibromobutane. The product tetrakis-[3-methoxy-4-(N-carbazole)pentyloxy]phenylporphyrin. Mass spectrum molecular ion peak: 1739. Elemental analysis according to chemical formula C 116 h 106 N 8 o 8 Calculated: C: 80.0%; H: 6.1%; N: 6.4%; O: 7.4%; found: C: 79.8%; H: 6.2%; N: 6.5%;
example 3
[0044] Example 3: Synthesis of Compound 1-1-03
[0045] The synthesis of compound 1-1-03 was the same as in Example 1. Just use 1,6-dibromohexane instead of 1,4-dibromobutane. The product tetrakis-[3-methoxy-4-(N-carbazole)hexyloxy]phenylporphyrin. Mass spectrum molecular ion peak: 1795. Elemental analysis according to chemical formula C 120 h 114 N 8 o 8 Calculated: C: 80.0%; H: 6.4%; N: 6.2%; O: 7.1%; Experimental values: C: 80.2%; H: 6.3%; N: 6.1%;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminescence peak | aaaaa | aaaaa |
| Shine | aaaaa | aaaaa |
| Luminescence peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com