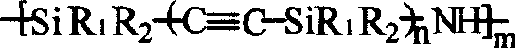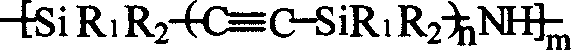Alkynyl-containing poly-siloxane and method for preparing same
A technology of alkynyl polysilazane and methyl, which is applied in the field of polysilazane and its preparation, and achieves the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In a 250ml three-necked flask, add 50ml tetrahydrofuran, 50ml diethyl ether and 50ml (n-BuLi, 2.5mol / L), under N 2 Under protection, a mixed solution of 36 ml of ether and 3.6 ml of trichlorethylene was slowly added dropwise to the mixed solution in the three-necked flask at -78°C. After the dropwise addition, the stirring was continued for 12 hours to obtain a white cloudy liquid of lithium acetylene.
[0020] Add 20ml ether and 0.1mol Me 2 SiCl 2 , N 2 Slowly drip the cloudy lithium acetylene solution under protection. After the dropwise addition was completed, stirring was continued at -30°C for 10 hours, and then left to settle. The system is layered, the upper layer is light yellow clear liquid, the lower layer is white precipitate, N 2 Protect the supernatant with a glass sand core under N 2 Press it into a clean and dry three-neck flask, and wash the precipitate three times with ether. Combine the filtrates. The solvent was removed from the filtrate under...
Embodiment 2
[0035] Add 50ml ether and 0.2mol MePhSiCl to a 500ml three-neck flask 2 , N 2 Slowly drip the cloudy lithium acetylene solution under protection. After the dropwise addition was completed, stirring was continued at 20° C. for 20 hours, and then left to settle. The system is layered, the upper layer is light yellow clear liquid, the lower layer is white precipitate, N 2Under protection, the supernatant was hydraulically poured into a clean and dry three-necked bottle with a glass sand core, and the precipitate was washed six times with ether. Combine the filtrates. The solvent was removed from the filtrate under reduced pressure to obtain the alkynyl-containing chlorosilane oligomer as a yellow viscous liquid with a yield of 93%.
[0036] Characterization of alkyne-containing chlorosilane oligomers:
[0037] 29 Si-NMR (ppm): -10.0 (Cl-SiMePh), -44.0 (C≡C-SiMePh-C≡C).
[0038] 2 g of alkynyl-containing chlorosilane oligomers were dissolved in 50 ml of xylene, stirred and ...
Embodiment 3
[0046] Add 100ml ether and 0.1molPh into a 500ml three-neck flask 2 SiCl 2 , N 2 Slowly drip the cloudy lithium acetylene solution under protection. After the dropwise addition was completed, stirring was continued at 0° C. for 12 hours, and the mixture was left to settle. The system is stratified, the upper layer is orange clear liquid, the lower layer is pink precipitate, N 2 Protect the supernatant with a glass sand core under N 2 Press it into a clean and dry three-neck flask, and wash the precipitate four times with ether. Combine the filtrates. The solvent was removed from the filtrate under reduced pressure to obtain the alkynyl-containing chlorosilane oligomer as a brown viscous liquid with a yield of 91%.
[0047] Characterization of alkyne-containing chlorosilane oligomers:
[0048] 29 Si-NMR (ppm): -20.0 (Cl-SiPh 2 ), -49.0 (C≡C-SiPh 2 -C≡C).
[0049] Dissolve 1 g of alkynyl-containing chlorosilane oligomer in 50 ml of benzene, and pass ammonia gas at 80°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com