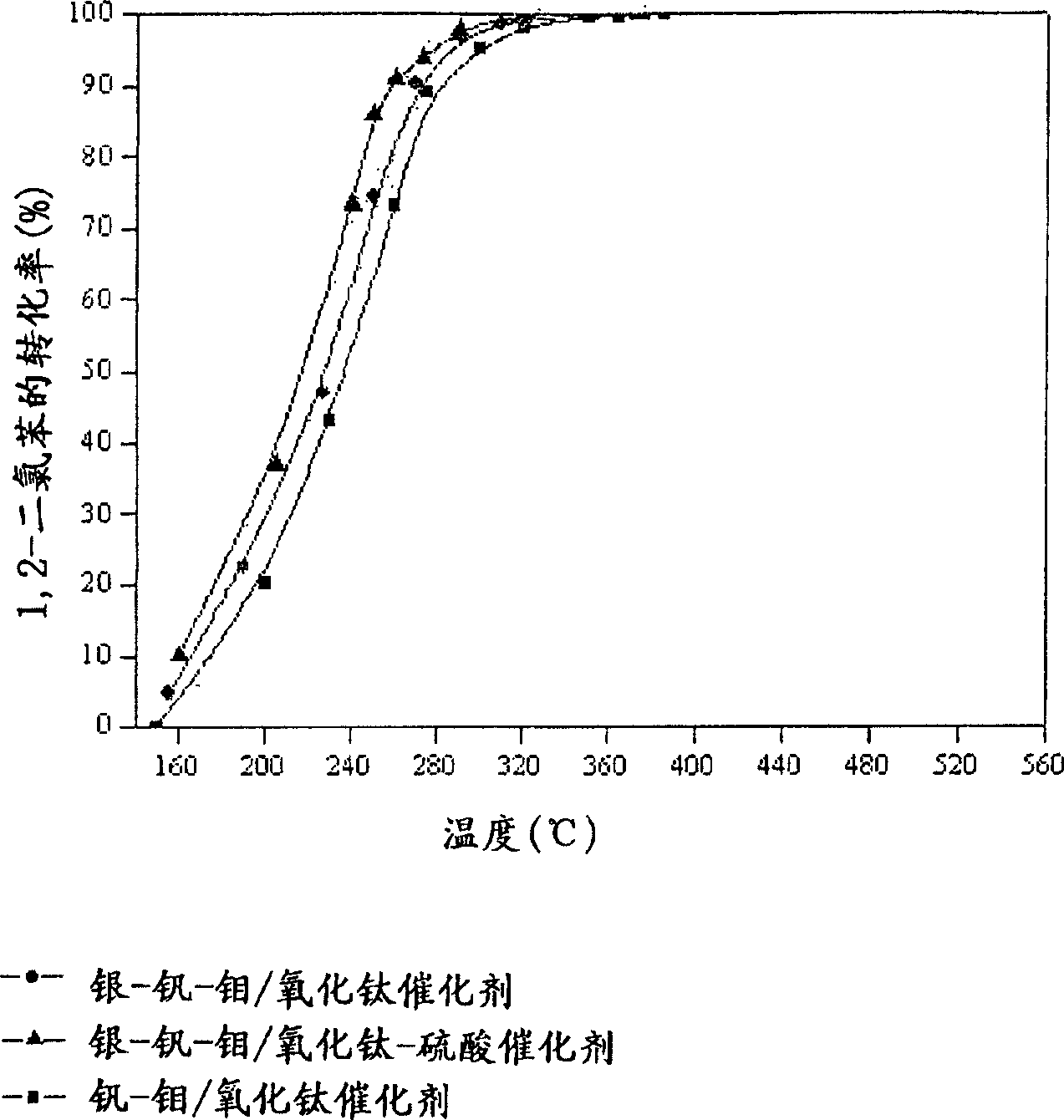
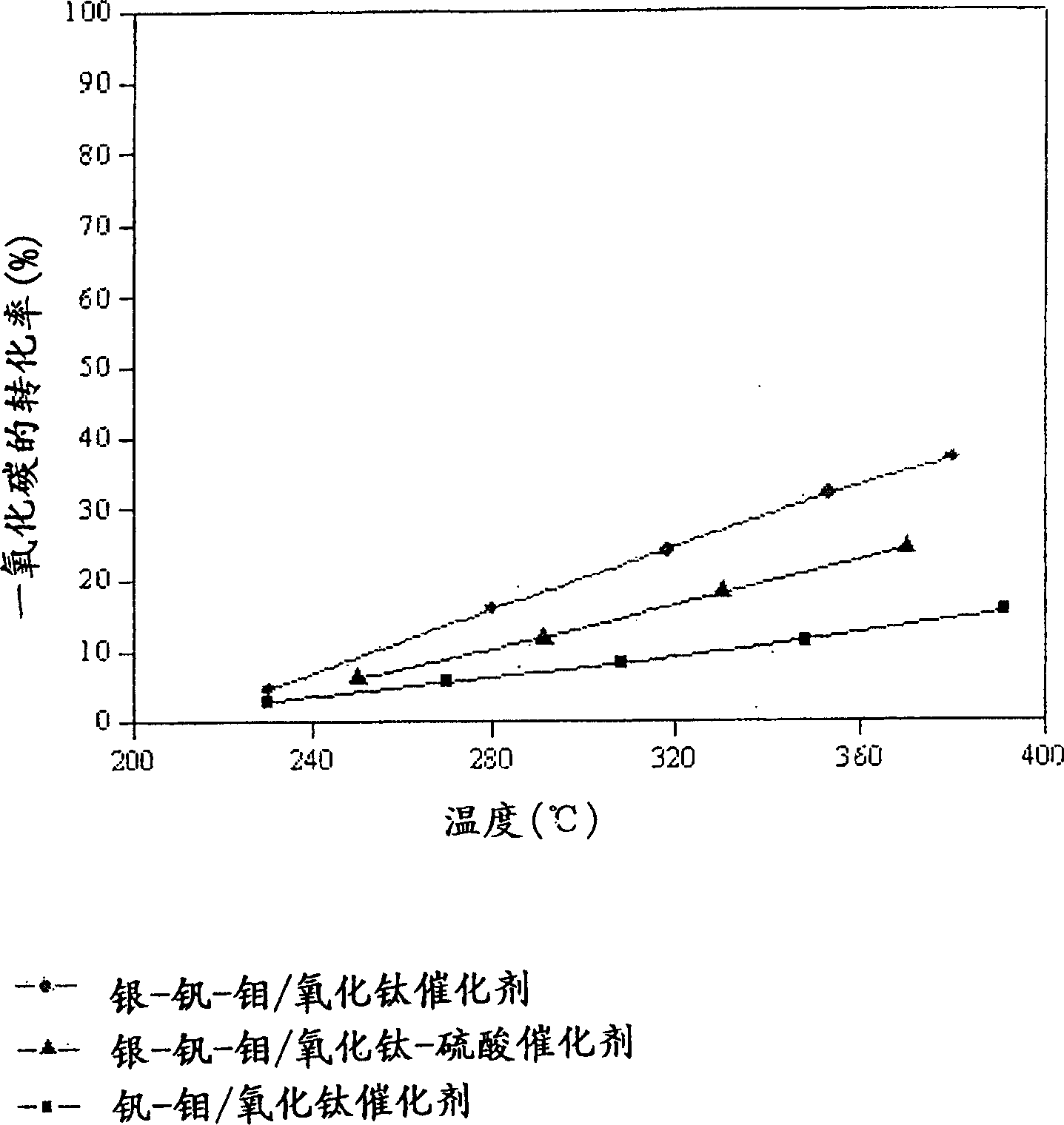
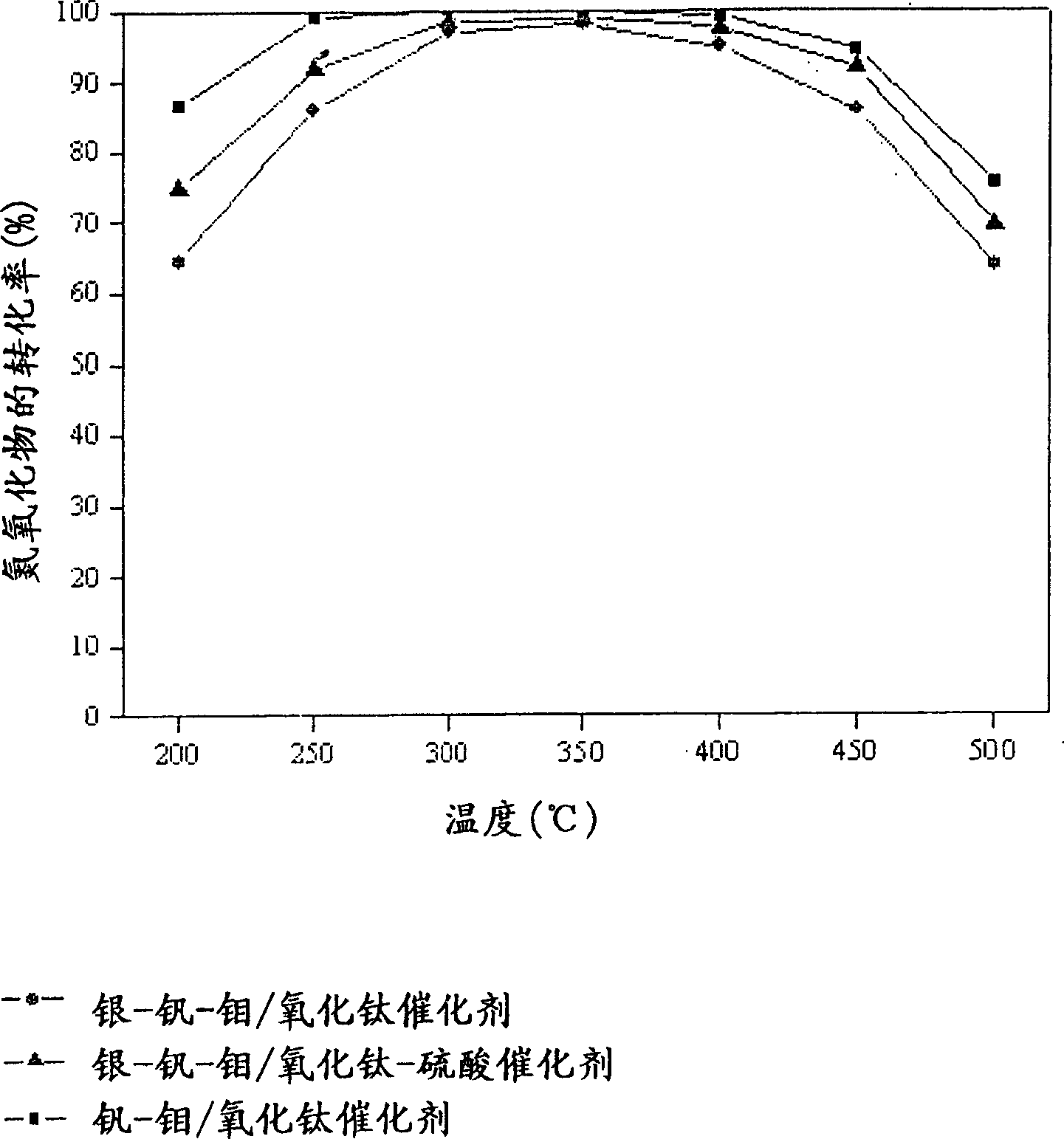
Catalyst for de-aromatic halogeno compound containing dioxina, carbon monoxide and nitrogen oxide and its use
A carbon monoxide and nitrogen oxide technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of reduced nitrogen oxide removal efficiency and catalyst availability Restrictions, price increases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Vanadium-molybdenum / titanium oxide catalyst [V-Mo / TiO 2 ] preparation
[0050] In deionized water, add oxalic acid (C 2 h 2 o 4 2H 2 O, produced by DUKSAN PURECHEMICALS CO., LTD., KOREA), after stirring the solution, ammonium metavanadate (NH 4 VO 3 , produced by Samchun Pure Chemical Co.Ltd., KOREA) and ammonium molybdate ((NH 4 )Mo 7 o 24 4H 2 O, produced by DUKSAN PURE CHEMICALS CO., LTD., KOREA) were charged together into the solution and then dissolved by vigorous stirring. As a procatalyst aqueous solution, titanium oxide DT51 (purchased from Millennium, FRANCE; with defined crystal structure - anatase 100%, specific surface area: 80 to 100 m 2 / g,SO 4 Content: 1 to 2 wt%), followed by vigorous stirring to produce a homogeneous slurry. Wherein, the materials such as titanium oxide, ammonium metavanadate, and ammonium molybdate are added in a weight ratio of 25.93:1:2.55 to generate the slurry. The prepared slurry is coated on the surface of the cordie...
Embodiment 2
[0052] Silver-vanadium-molybdenum / titanium oxide catalyst [Ag-V-Mo / TiO 2 ] preparation
[0053] The catalyst produced in Example 1 was prepared in silver nitrate (AgNO 3 , Han-Gyeul Gold Co., produced by KOREA), and then naturally dried overnight. Then the catalyst was dried at 120° C. for 4 hours, then the temperature was raised to 500° C. at 10° C. / min, and the catalyst was calcined at the same temperature for 2 hours to obtain the desired silver-vanadium-molybdenum / titanium oxide catalyst. The catalyst produced contained 0.84% by weight of vanadium and 5.33% by weight of molybdenum and 2% by weight of silver (based on titanium oxide).
Embodiment 3
[0055] Silver-vanadium-molybdenum / titanium oxide-sulfuric acid catalyst [Ag-V-Mo / TiO 2 -SO 4 2- ] preparation
[0056] The catalyst produced in embodiment 2 was dissolved in 0.21M sulfuric acid aqueous solution (H 2 SO 4 · xSO 3 , Aldrich, produced in USA) and then dried naturally overnight. The catalyst was then dried at 120° C. for 4 hours, and then the temperature was raised to 500° C. at a rate of 10° C. / min, and the catalyst was calcined to obtain the desired silver-vanadium-molybdenum / titanium oxide-sulfuric acid catalyst. The catalyst produced contained 0.84% by weight vanadium and 5.33% by weight molybdenum, 2% by weight silver and 2% by weight sulfate (based on titanium oxide).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com