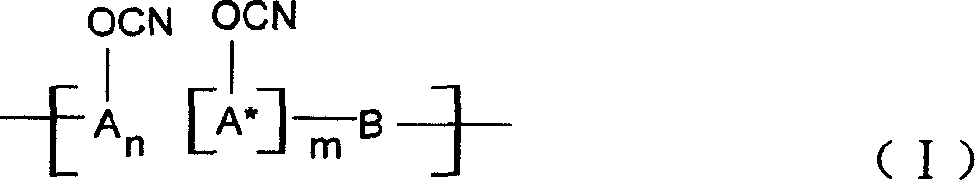
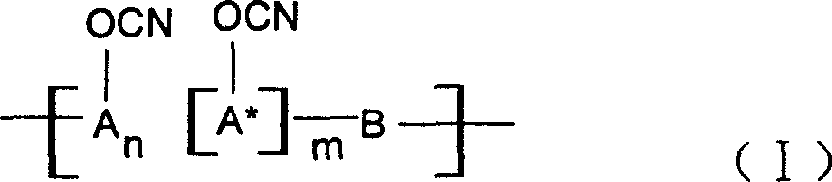
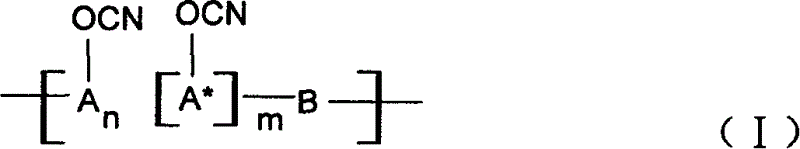Ablative material of resin of phenolic cyanate
A technology of phenolic cyanate ester and ablation material, applied in the field of high temperature resistant ablation material, can solve the problems of low carbon content, high melt viscosity and high content of heteroatoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: synthetic novolac cyanate
[0027] A 37% aqueous solution of phenol and formaldehyde and oxalic acid in a molar ratio of 1:0.9:0.005 are put into a three-necked flask equipped with a reflux condenser, reacted at 80-100°C for 3-5 hours, washed with water, and vacuumized to obtain the softening point The novolac between 40 and 60°C, the novolac is dissolved in the mixed solvent of dichloromethane and ethyl acetate, put into the four-necked bottle together with BrCN, drop triethylamine, phenolic hydroxyl group and BrCN and triethylamine The molar ratio is 1:1.05:1.10, react at -15°C to -5°C for 5 hours, separate the salt and solvent, and perform purification treatment to obtain novolac cyanate. In this example n=2~5, m=0.
[0028] 1-2: Using the same method as 1-1, change phenol to p-phenylphenol to obtain novolac cyanate. In this example n=2~5, m=0.
[0029] 1-3: Using the same method as 1-1, change phenol to o-phenylphenol to obtain novolac cyanate. In ...
Embodiment 2
[0031] Embodiment 2: Synthesis of linear fused ring phenolic cyanate
[0032] 2-1: A 37% aqueous solution of 1-naphthol and formaldehyde and oxalic acid in a molar ratio of 1:0.80:0.005 are put into a three-necked flask equipped with a reflux condenser, in the presence of a ketone solvent, at 60-100°C React for 3 to 5 hours, wash with water, and vacuumize to obtain linear condensed ring phenol novolac with a softening point between 40 and 60°C. Dissolve linear fused-ring phenolic aldehyde in dichloromethane solvent, put it into a four-necked flask together with BrCN, add triethylamine dropwise, the molar ratio of phenolic hydroxyl group to BrCN and triethylamine is 1:1.05:1.10, and store it at -15°C~ React at -5°C for 5 hours, separate the salt and solvent, and perform purification treatment to obtain linear fused ring phenol novolac cyanate. In this example n=0, m=2~5.
[0033] 2-2: Using the same method as 2-1, use 2-naphthol and polyoxymethylene as raw materials to obtain...
Embodiment 3
[0035] Embodiment 3: synthetic copolymerization type phenolic cyanate
[0036] 3-1: 37% aqueous solution of phenol, naphthol (molar ratio is 1: 1) and formaldehyde and oxalic acid according to phenol, aldehyde, acid molar ratio 1: 0.9: 0.005, drop in the three-necked flask that is equipped with reflux condenser, React at 80-100°C for 3-5 hours, wash with water, and vacuumize to obtain copolymerized phenolic formaldehyde. The copolymerized phenolic formaldehyde is dissolved in acetone solvent, put into a four-necked bottle with BrCN, and triethylamine, phenolic hydroxyl group and BrCN are added dropwise. And the molar ratio of triethylamine is 1:1.05:1.10, react at -15°C to -5°C for 5 hours, separate the salt and solvent, and perform purification treatment to obtain copolymerized phenolic cyanate. In this example n:m=1:1.
[0037] 3-2: Change the molar ratio of phenol and naphthol to 1:2, and obtain the copolymerized phenolic novolac cyanate in the same way as 3-1 with benzald...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com