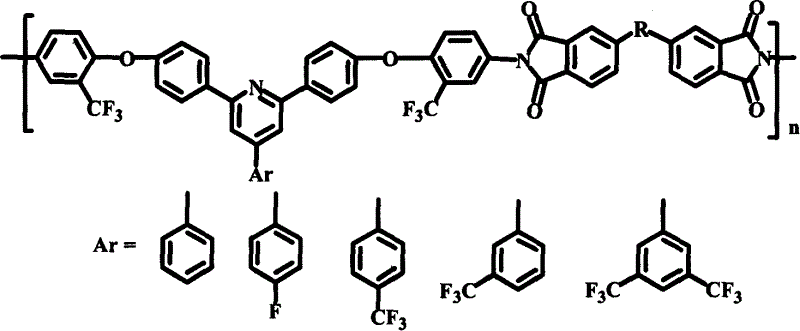
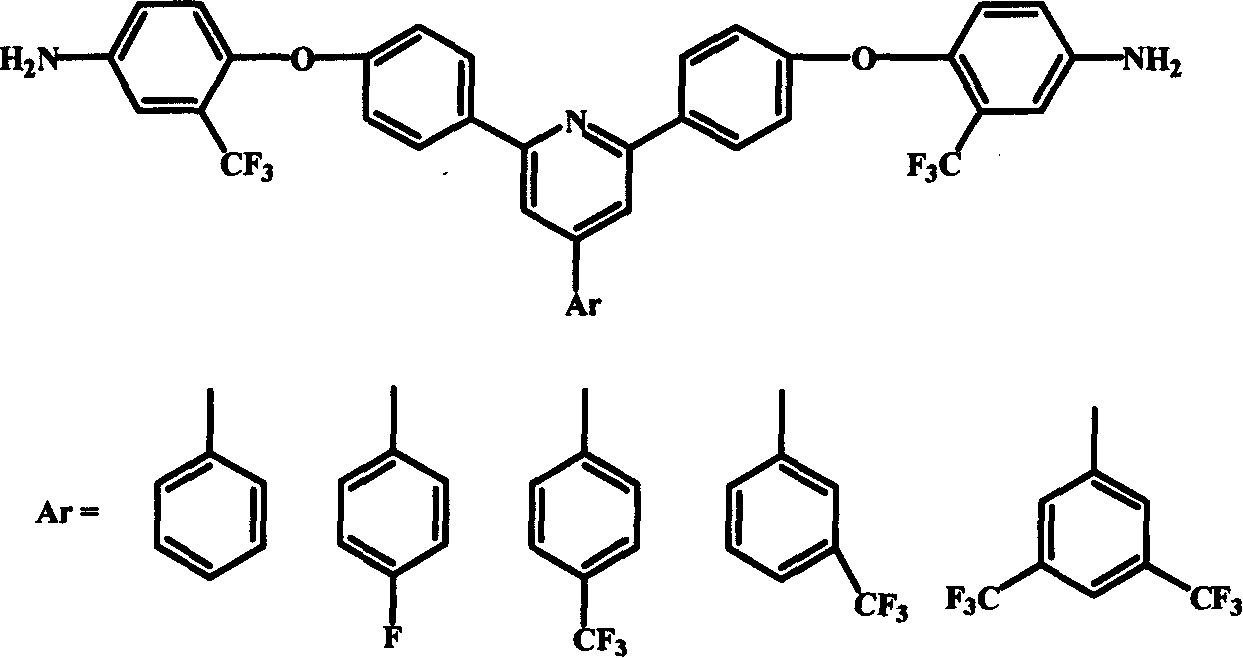
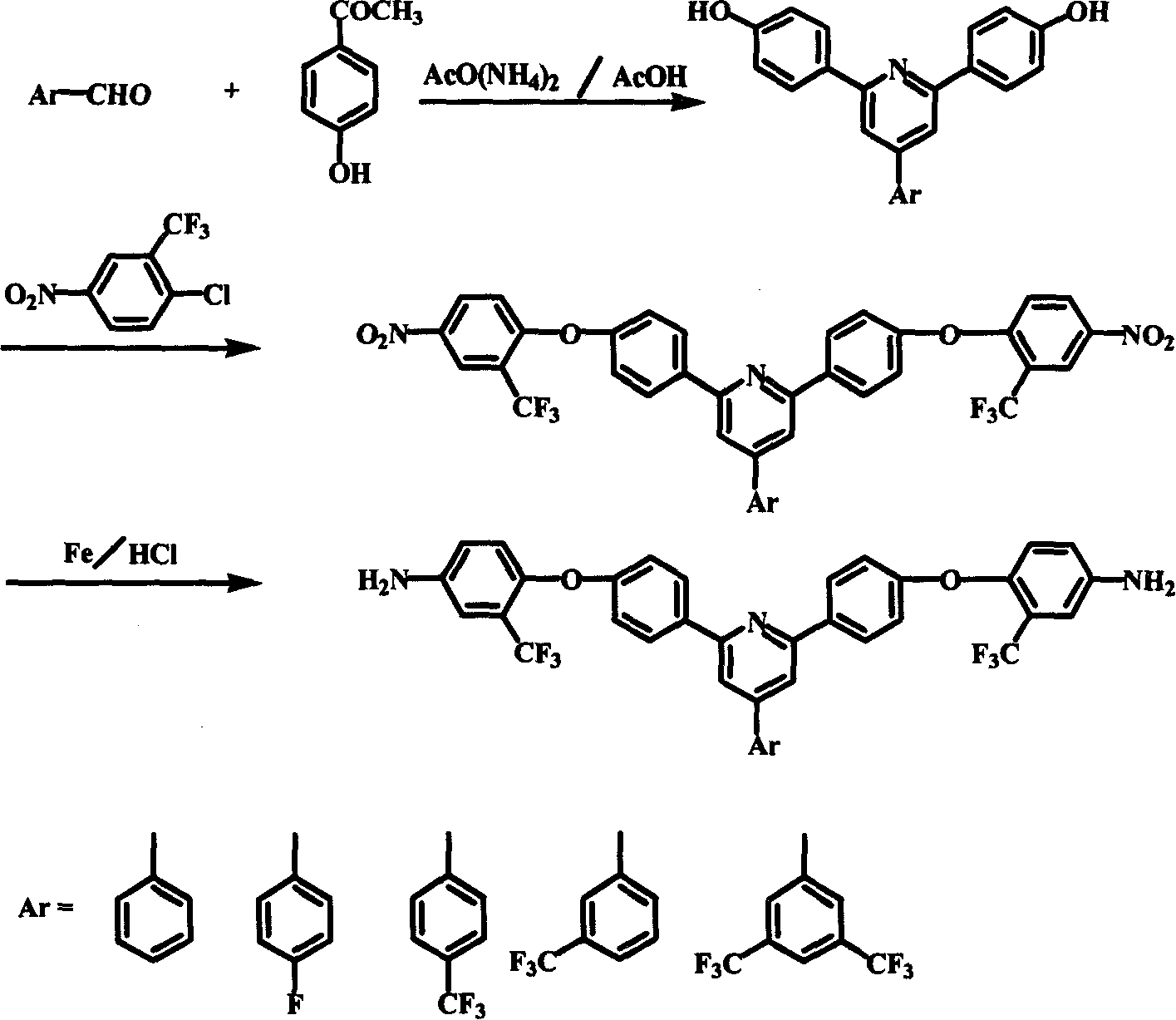
Polyimide material, and its preparing method and use
A polyimide and polyamic acid technology, applied in the field of polyimide materials, can solve the problem of reducing the reactivity of aromatic diamines, low dielectric constant glass transition temperature, and difficulty in obtaining high molecular weight polyimide polymerization In order to achieve the effects of excellent solubility, excellent mechanical properties and electrical insulation properties, and excellent transparency of visible light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] 4-phenyl-2,6 bis(4-hydroxy-phenyl)-pyridine: In the three-necked flask equipped with mechanical stirring, condenser and nitrogen inlet and outlet, add 530 parts of benzaldehyde and 250 parts of p-hydroxyacetophenone , 1000 parts of ammonium acetate, 2000ml of glacial acetic acid. The reaction mixture was refluxed for 4-6 hours with stirring to obtain a homogeneous orange-red solution, which was poured into 4000 ml of water to obtain a yellow-brown precipitate. Collect the precipitate by filtration and wash it with water 3 times to obtain the crude product; dissolve the crude product in 1000ml 2 / 1 tetrahydrofuran / petroleum ether mixed solvent, collect the filtrate by filtration; freeze the filtrate overnight in the refrigerator below -15°C, and filter to obtain light yellow Powdered 4-phenyl-2,6-bis(4-hydroxy-phenyl)-pyridine.
[0032]4-phenyl-2,6-bis(4-(4-nitro-2-trifluoromethyl-phenoxy)-phenyl)pyridine: 100 parts of 4-phenyl-2,6-bis(4 -Hydroxy-phenyl)-pyridine and 30...
example 2
[0035] 4-(4-trifluoromethylphenyl)-2,6 bis(4-hydroxyl-phenyl)-pyridine: Add p-trifluoro 1050 parts of methyl benzaldehyde, 300 parts of p-hydroxyacetophenone, 1000 parts of ammonium acetate, 1200 ml of glacial acetic acid. The reaction mixture was refluxed for 4-5 hours with stirring to obtain a homogeneous orange-red solution, which was poured into 6000 ml of water to obtain a yellow-brown precipitate. The precipitate was collected by filtration and washed with water to obtain a crude product; recrystallized from acetone to obtain light yellow powder 4-(4-trifluoromethylphenyl)-2,6bis(4-hydroxy-phenyl)-pyridine.
[0036] 4-(4-trifluoromethylphenyl)-2,6-bis(4-(4-nitro-2-trifluoromethyl-phenoxy)-phenyl)pyridine: 240 parts of 4-( 4-trifluoromethylphenyl)-2,6 bis(4-hydroxy-phenyl)-pyridine and 60 parts of potassium hydroxide, 1000ml dimethyl sulfoxide, 600ml toluene were added equipped with mechanical stirring, thermometer, reflux condensation Pipe, water separator and three-ne...
example 3
[0039] 4-(3', 5'-bistrifluoromethylphenyl)-2,6 bis(4-hydroxyl-phenyl)-pyridine: in a three-neck flask equipped with mechanical stirring, condenser and nitrogen inlet and outlet, Add 1200 parts of 3',5'-bistrifluoromethylbenzaldehyde, 250 parts of p-hydroxyacetophenone, 1000 parts of ammonium acetate and 2000 ml of glacial acetic acid. The reaction mixture was refluxed for 4-6 hours under stirring to obtain an orange-red homogeneous clear solution, which was poured into 5200 ml of water to obtain a yellow-brown precipitate. The precipitate was collected by filtration and washed 3 times with water to obtain a crude product; the crude product was recrystallized from a mixed solvent of diethyl ether / petroleum ether to obtain light yellow powder 4-(3',5'-bistrifluoromethylphenyl)-2 , 6 bis(4-hydroxy-phenyl)-pyridine.
[0040] 4-(3', 5'-bistrifluoromethylphenyl)-2,6-bis(4-(4-nitro-2-trifluoromethyl-phenoxy)-phenyl)pyridine: the 230 parts of 4-phenyl-2,6 bis(4-hydroxy-phenyl)-pyrid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com