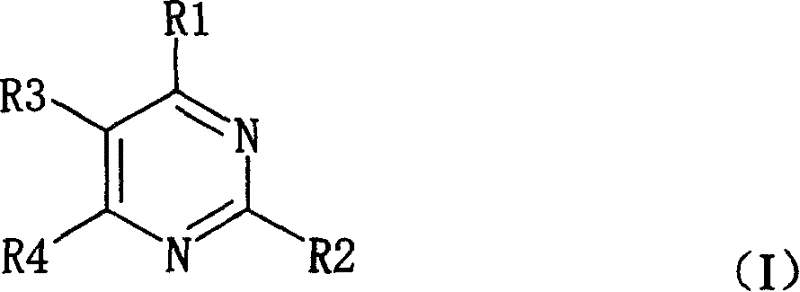
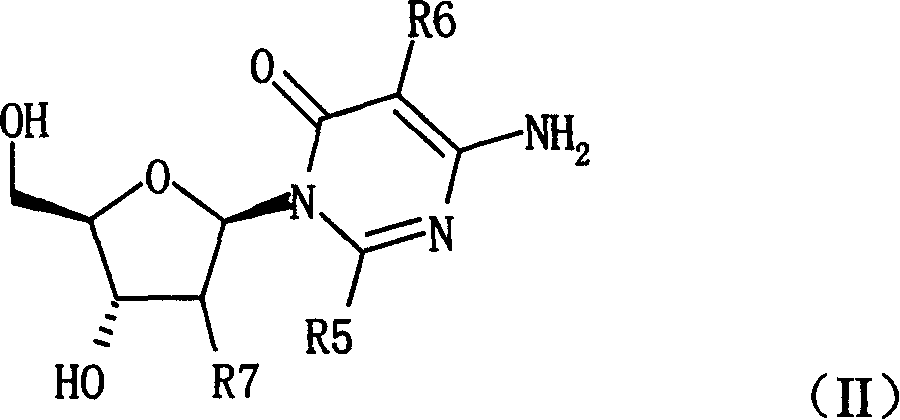
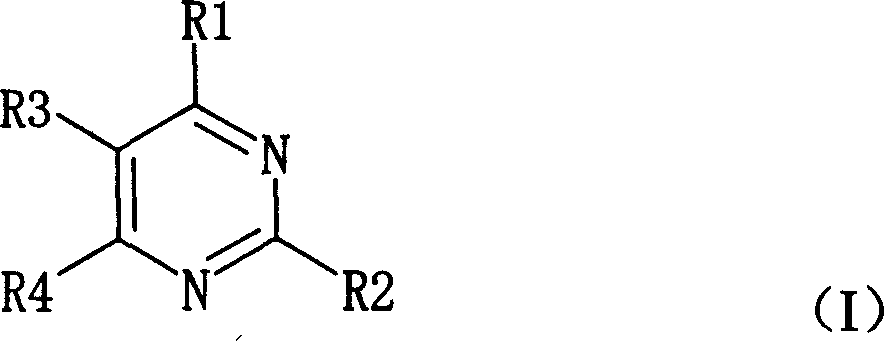
Pyrimidine nucleosides preparation method and novel pyrimidine nucleoside
A technology for pyrimidine nucleoside compound and cytidine nucleoside phosphorylase activity, applied in the field of preparation of pyrimidine nucleoside compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0053] The following examples illustrate the present invention, but the enumerated examples do not limit the scope of protection of the present invention.
[0054] The identification of the generated pyrimidine nucleoside compound was carried out as follows: the reaction solution was ultrafiltered and purified with a silica gel column, the product was extracted and vacuum-dried, and the obtained product was analyzed by C13-NMR and H1-NMR.
[0055] In addition, the generated pyrimidine nucleoside compounds were quantitatively analyzed by high performance liquid chromatography (HPLC). The analysis conditions are as follows:
[0056] Chromatographic column: Develosil ODS-MG-5, 4.6×250mm (Nomura Chemical)
[0057] Column temperature: 40°C
[0058] Pump flow rate: 1.0ml / min
[0059] Detection: UV 254nm
[0060] Eluent: potassium dihydrogen phosphate (50mM): methanol = 8: 1 (V / V)
reference example 1
[0061] Reference Example 1 (Preparation of cells producing cytidine phosphorylase)
[0062] Escherichia coli chromosomal DNA was prepared as follows:
[0063] Escherichia coli K-12 / XL-10 strain (Stratagene Company) was implanted into 50 ml of LB culture medium, cultivated overnight at 37° C., and then the colony was collected and treated with a lysozyme-containing 1 mg / ml lysozyme. After treating the lysate with phenol, the DNA was precipitated with ethanol by a usual method. The resulting DNA precipitate was wound and precipitated along the glass rod, recovered and washed, and used for PCR.
[0064] Oligonucleotides (synthesized by Hokkaido System Science Co., Ltd.) having base sequences in SED ID NOs: 1, 2, respectively, were used as primers for PCR. These nucleotide sequences were designed based on the nucleotide sequence of the deoD gene encoding the known purine nucleoside phosphorylase of Escherichia coli (the number in the gene bank is AE000508 (the base number of the...
reference example 2
[0069] Reference Example 2 (Preparation of Purified Cytidine Phosphorylase)
[0070]The bacteria in the suspension prepared in Reference Example 1 were destroyed with an ultrasonic crusher. Heat treatment at 70°C for 10 minutes, and then centrifuge to obtain a crude enzyme solution, which is then injected into a DEAE-Toyopearl (3cm×3cm) chromatographic column manufactured by Toso, which has been washed with 50mM Tris-HCl buffer (pH 7.5) balanced. Then it was eluted with a linear gradient with 50mM-500mM NaCl solution to collect active fractions. The eluate was saturated with 70% aqueous ammonium sulfate to form a precipitate for recovery. The precipitate was dialyzed against 10 mM phosphate buffer (pH 7.5). The dialyzed solution was injected into a chromatographic column loaded with hydroxyapatite (3 cm×15 cm), which had been equilibrated with 10 mM phosphate buffer (pH 7.5). It was then eluted with 10mM-50mM 10mM phosphate buffer (pH 7.5) to collect active fractions. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com