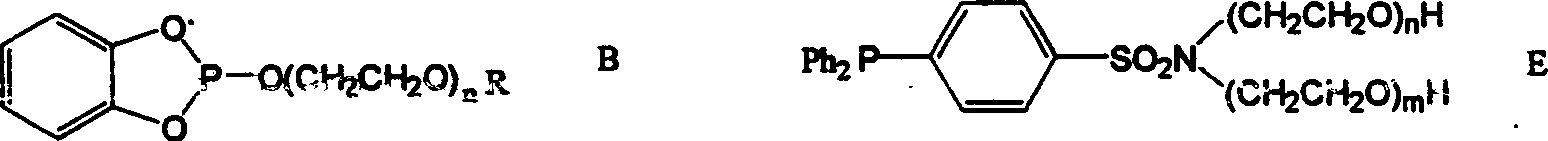Poly ethyelen glycol two-phase system and application
A polyethylene glycol, system technology, applied in the direction of carbon monoxide reaction preparation, organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Phosphine ligand (A)P[O(CH 2 CH 2 O)nCH 3 ] 3 Synthesis
[0023] 44.7mmol average molecular weight of 150, 350, 750, 1150 polyethylene glycol monomethyl ether CH 3 (OCH 2 CH 2 ) m OH, 47.85mmol of triethylamine and 100ml of toluene were placed in a 250ml three-necked flask equipped with mechanical stirring, and 14.5mmol of PCl was dissolved under stirring after cooling to 0°C. 3 40ml of toluene solution was slowly dripped from the dropping funnel within 120 minutes, and the ice-water bath was removed after dripping, and stirring was continued for 9 hours at room temperature.
[0024] in N 2 Under protection, the reaction mixture was filtered, triethylamine hydrochloride was filtered off, and the filtrate was 2 The solvent toluene was vacuumed off under protection to obtain a colorless viscous liquid. NMR analysis data is H 1 NMR (δ, C 6 D. 6 ), δ3.904-3.859(m, 6H), 3.412-3.378(m, 24H), 3.052(S, 9H); 13 CNMR (δ, C 6 D. 6 ), 72.912, 71.971, 71.926, 71.561,...
Embodiment 2
[0026] RhCl 3 / P[O(CH2 CH 2 O)nCH 3 ] 3 (Phosphine Ligand A) Catalyzed Hydroformylation of PEG Two-phase Isobutyl Styrene
[0027] In a 100ml stainless steel autoclave, place 4.0g PEG-4000, 3.0g n-heptane, 3.5g toluene, 1.7g (10mmol) p-isobutylstyrene, internal standard decane and a certain amount of RhCl 3 and Phosphine Ligand A (M=150). Tighten the autoclave, use 2.0MPa synthesis gas (CO / H 2 =1:1) to replace three times and fill to the required reaction pressure. Under the reaction conditions that the temperature is 120°C, the pressure is 5.0MPa, the substrate: rhodium = 1000: 1, and P / Rh = 13, after 2 hours of reaction, after cooling to room temperature, the pressure is released, the lid of the kettle is opened, and the upper layer is separated According to GC analysis of the reaction solution, the conversion rate of p-isobutylstyrene is 100%, and the yield of aldehyde is 95-97%.
Embodiment 3
[0029] Catalyst Separation and Recycling Activity Investigation
[0030] The method is shown in Example 2, the difference is that "re-add 1.7g p-isobutylstyrene" (IBS), 3.5g toluene, 3.0g n-heptane and internal standard decane for each cycle. The experimental results are shown in Table 1.
[0031] Cycles
[0032] As can be seen from Table 1, the catalyst was recycled 7 times, and its activity was basically unchanged, but the aldehyde yield had a certain decline. After 8 cycles, the activity and aldehyde yield had a certain decline. After 8 cycles, the activity and aldehyde yield rates have a downward trend.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com