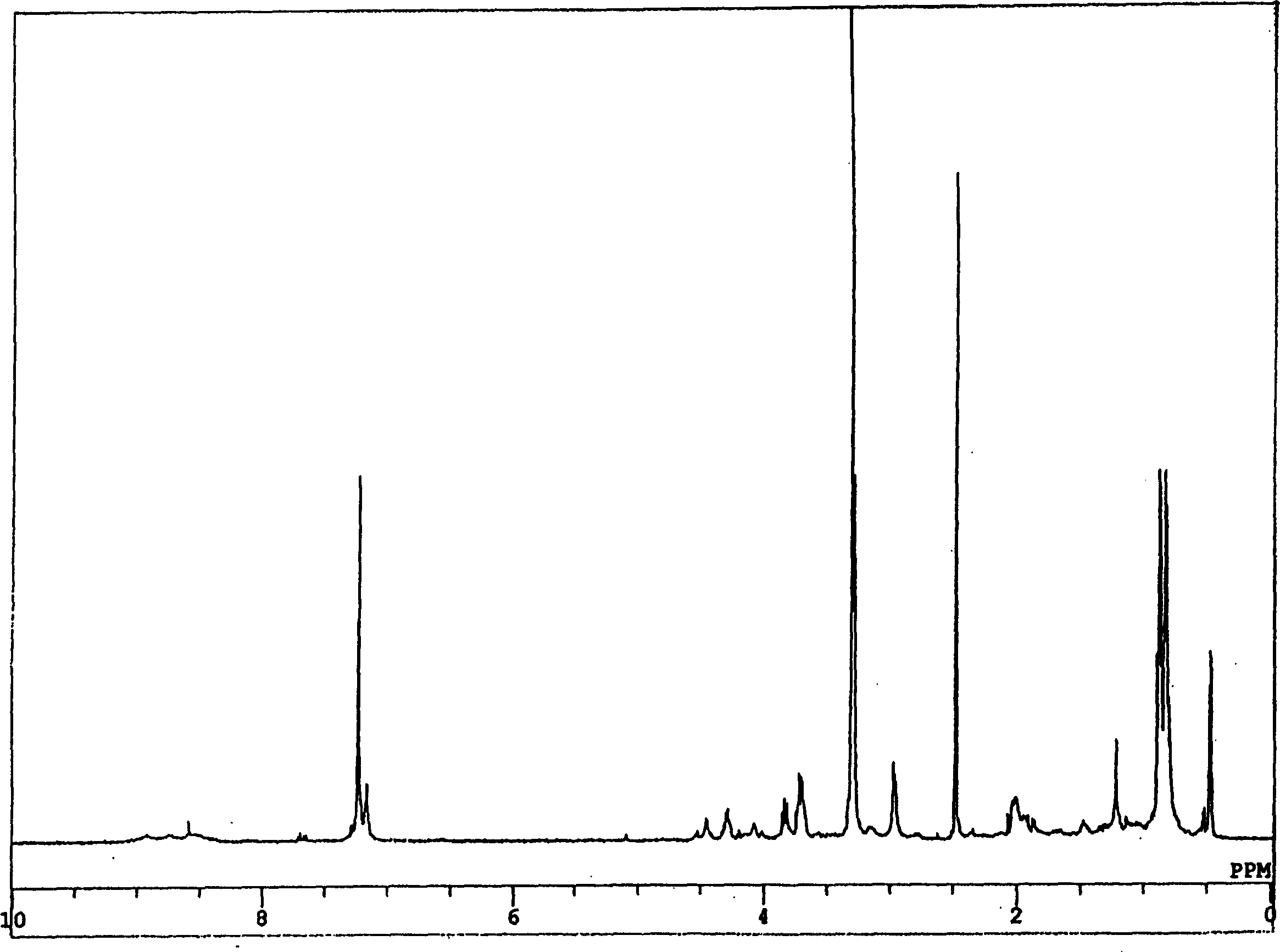
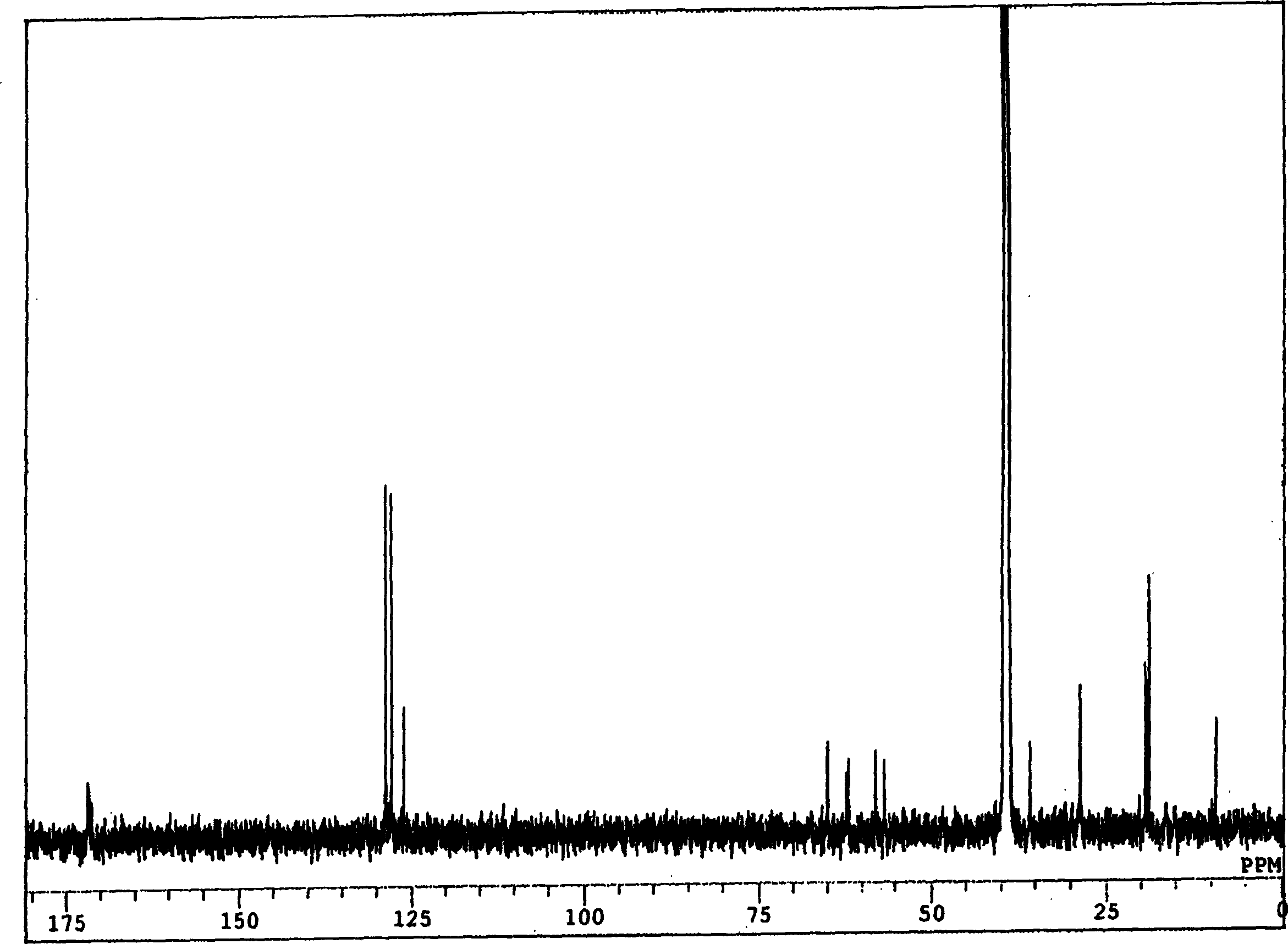
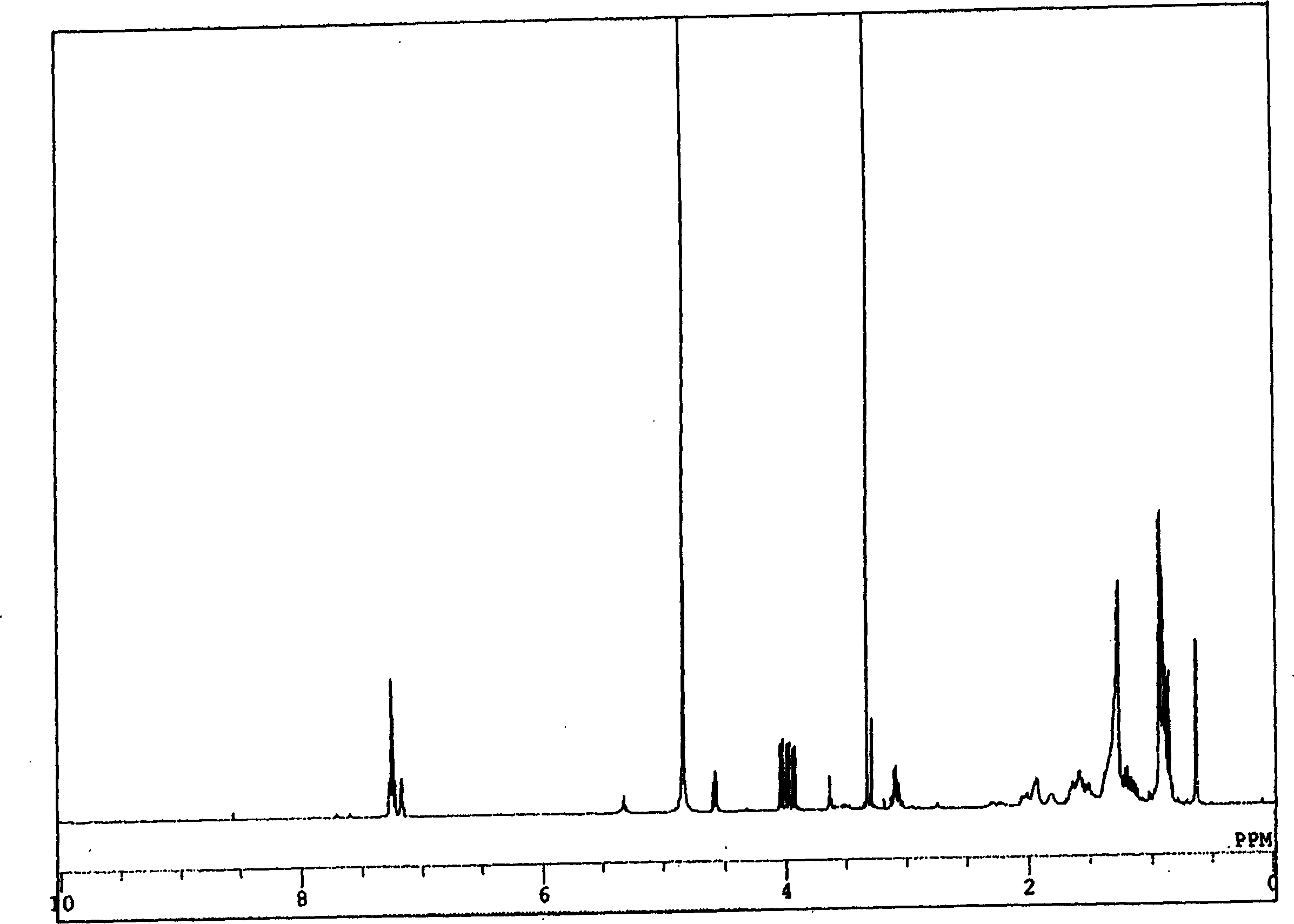
New compound with cativity for inhibiting glycine transport factor
A technology for inhibiting activity and glycine, applied in drug combinations, chemical instruments and methods, cyclic peptide components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Example 1 Preparation of WSS2217, WSS2218, WSS2219 and WS2220.
[0145] 1. Put the liquid culture medium containing 2.5% soluble starch, 1% glucose, 0.5% fish meal, 0.3% cottonseed meal, 0.3% NZ CASE, 0.2% yeast juice, 0.2% calcium carbonate in the Erlenmeyer flask, at 121°C , Sterilize for 20 minutes. Then, the Nonomuraea sp. TA-0426 strain was inoculated on the sterile medium, and cultured with shaking at 28° C. and 200 rpm for three days as a seed culture solution.
[0146] Then, 100ml of sterile liquid medium composed of 2% corn flour, 1.0% glycerol, 0.5% glucose, 0.5% cottonseed meal, 0.5% wort juice, 0.3% polyptone, 0.05% magnesium sulfate and 0.3% calcium carbonate Pack into a 500ml Erlenmeyer flask. 4 ml of the aforementioned seed culture solution was added thereto to prepare 100 bottles, and cultured with shaking at 28° C. and 180 rpm for 14 days.
[0147] After the cultivation, 10 L of the obtained culture solution was added to 5 L of n-butanol, stirred, ce...
Embodiment 2
[0152] The preparation of embodiment 2 WSS2221 and WSS2222
[0153] 1. Add 60ml of liquid culture medium containing 2.5% soluble starch, 1% glucose, 0.5% fish meal, 0.3% cottonseed meal, 0.3% NZ CASE, 0.2% yeast juice and 0.2% calcium carbonate into the Erlenmeyer flask, 121°C, Sterilize for 20 minutes. Then, the Nonomuraea sp. TA-0426 strain was inoculated on the sterile medium, and cultured with shaking at 28° C. and 200 rpm for three days as a seed culture solution.
[0154] Then, 100 ml of sterile liquid culture medium composed of 2% corn flour, 1.0% glycerol, 0.5% glucose, 0.5% cottonseed meal, 0.5% wort juice, 0.3% polyptone, 0.05% magnesium sulfate and 0.3% calcium carbonate Pack into a 500ml Erlenmeyer flask. 4 ml of the aforementioned seed culture solution was added thereto to prepare 150 bottles, and cultured with shaking at 28° C. and 180 rpm for 14 days.
[0155] After the cultivation, 15 L of the obtained culture solution was added to 5 L of n-butanol, stirred ...
Embodiment 3
[0158] Embodiment 3 Preparation of WSS8027
[0159] Under nitrogen flow, 1 ml of dimethylformamide was added to sodium hydroxide (100.6 mg), cooled and stirred. 1 ml of dimethylformamide in which WSS2219 (12.9 mg) was dissolved was added thereto, and methyl iodide (300 ml) was added thereto, followed by stirring at room temperature for 1 hour. After the reaction was completed, water and ethyl acetate were added under cooling in an ice bath, and the ethyl acetate layer was concentrated under reduced pressure. The concentrate was separated by high-performance liquid chromatography [mobile phase: acetonitrile-0.02% trifluoroacetic acid aqueous solution (80:20), device: Watt Company, chromatography column: ODS-AM (Φ10×150mm) manufactured by Vaem Company] , detected at 210nm, the flow rate is 2.5ml / min. The separated components were concentrated to dryness under reduced pressure to obtain 4.4 mg of WSS8027.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com