Methyl analogs of simvastatin as novel HMG-CoA reductase inhibitors
A technology of simvastatin and methyl, applied in the field of novel methyl analogs, can solve problems such as unreported mevalonate analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
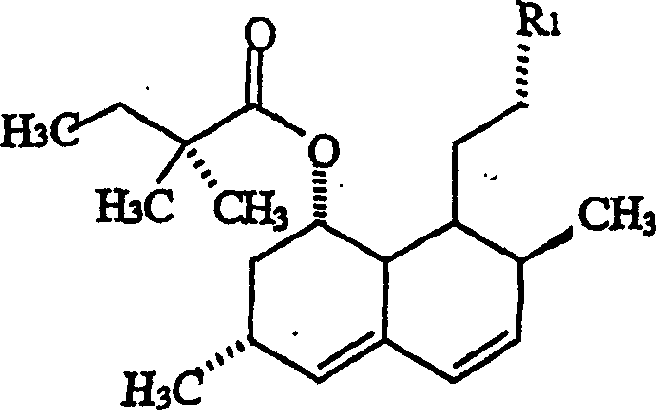
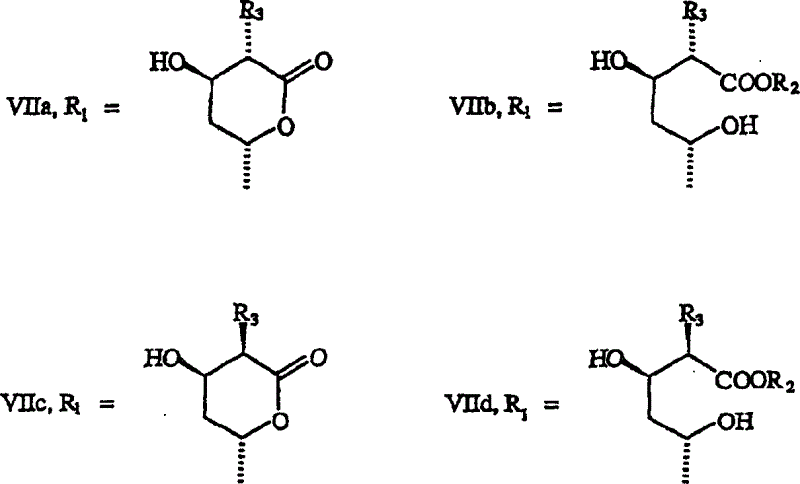
[0053] 6(R)-[2-[8(S)-[[2,2-dimethylbutyryl]oxy]-2(S),6(R)-dimethyl-1,2,6, 7,8,8a(R)-hexahydro-1(S)-naphthyl]ethyl]-3(S)-methyl, 4(R)-hydroxyl-3,4,5,6-tetrahydro- 2H-pyran-2-one (VIIc)
[0054] Lithium hexamethyldisilazane (0.3M, dissolved in hexane, 55.14ml, 0.05mol) was added via syringe to a THF solution (130ml) of simvastatin (10g, 0.023mol), and the above process was carried out under nitrogen , the temperature is maintained at -40°C to -45°C. The mixture was stirred at this temperature for 1 hour. Iodomethane (3.72 g, 0.026 mol) was added via syringe keeping the temperature below -30°C. The mixture was stirred at -40 to -45°C for a further 1 hour and quenched by adding water (50ml). The layers were separated and the aqueous layer was extracted with ethyl acetate (50ml x 2). The combined organic layers were washed with pre-cooled 2N hydrochloric acid (25ml) and then with water (30ml). The organic layer was concentrated to an oil which was crystallized from ethyl acet...
Embodiment 2
[0058] 6(R)-[2-[8(S)-[[2,2-dimethylbutyryl]oxy]-2(S),6(R)-dimethyl-1,2,6, 7,8,8a(R)-hexahydro-1(S)-naphthyl]ethyl]-3(R)-methyl, 4(R)-hydroxyl-3,4,5,6-tetrahydro- 2H-pyran-2-one (VIIa)
[0059] To a THF solution (15 ml) of pyrrolidine (2.73 g, 0.038 mol) was added N-butyllithium (1.6 M, 23 ml, 0.036 mol) via syringe at -30 to -25 °C under nitrogen. The mixture was stirred at -25°C for 30 minutes, and simvastatin solution (5 g, 0.012 mol) was added at -40°C. The mixture was stirred at -30°C for about 1 hour and iodomethane (5.12 g, 0.036 mol) was added. The mixture was stirred for a further 1 hour at -30°C and then for 20 minutes at -15°C. The reaction was quenched by adding water (50ml). The layers were separated and the aqueous layer was extracted with ethyl acetate (20ml). The combined organic layers were washed with 1N hydrochloric acid (30ml) and evaporated in vacuo to give an oil (4.2g). Assays were performed by HPLC and the oil was found to be a mixture of (VIIa) an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com