Large ring lactone antibiotic and preparing method thereof
A technology for macrolides and antibiotics, which is applied in the field of new macrolide antibiotics and their preparation, and can solve problems such as unsatisfactory absorption, distribution, metabolism, and unstable structure of macrolides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
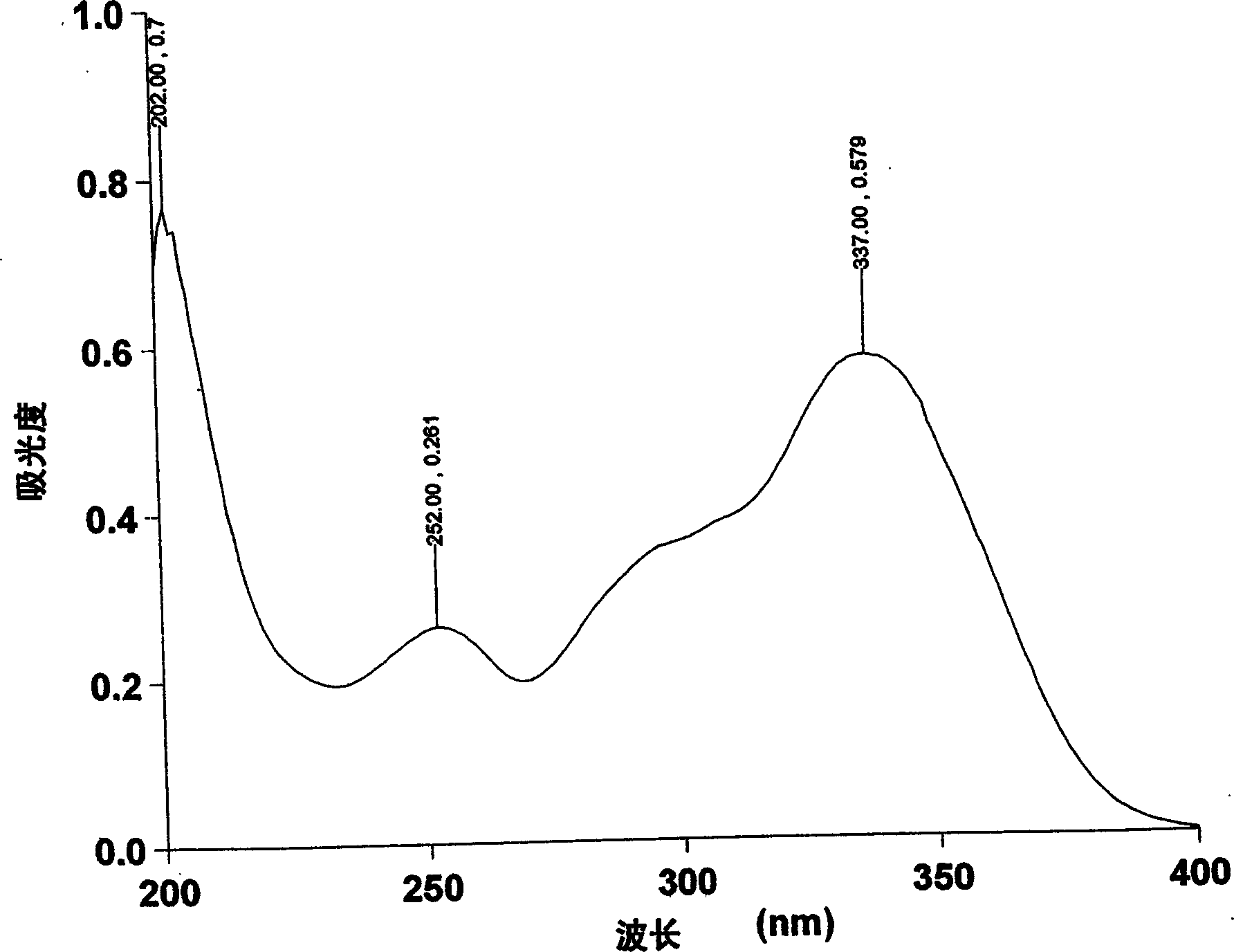
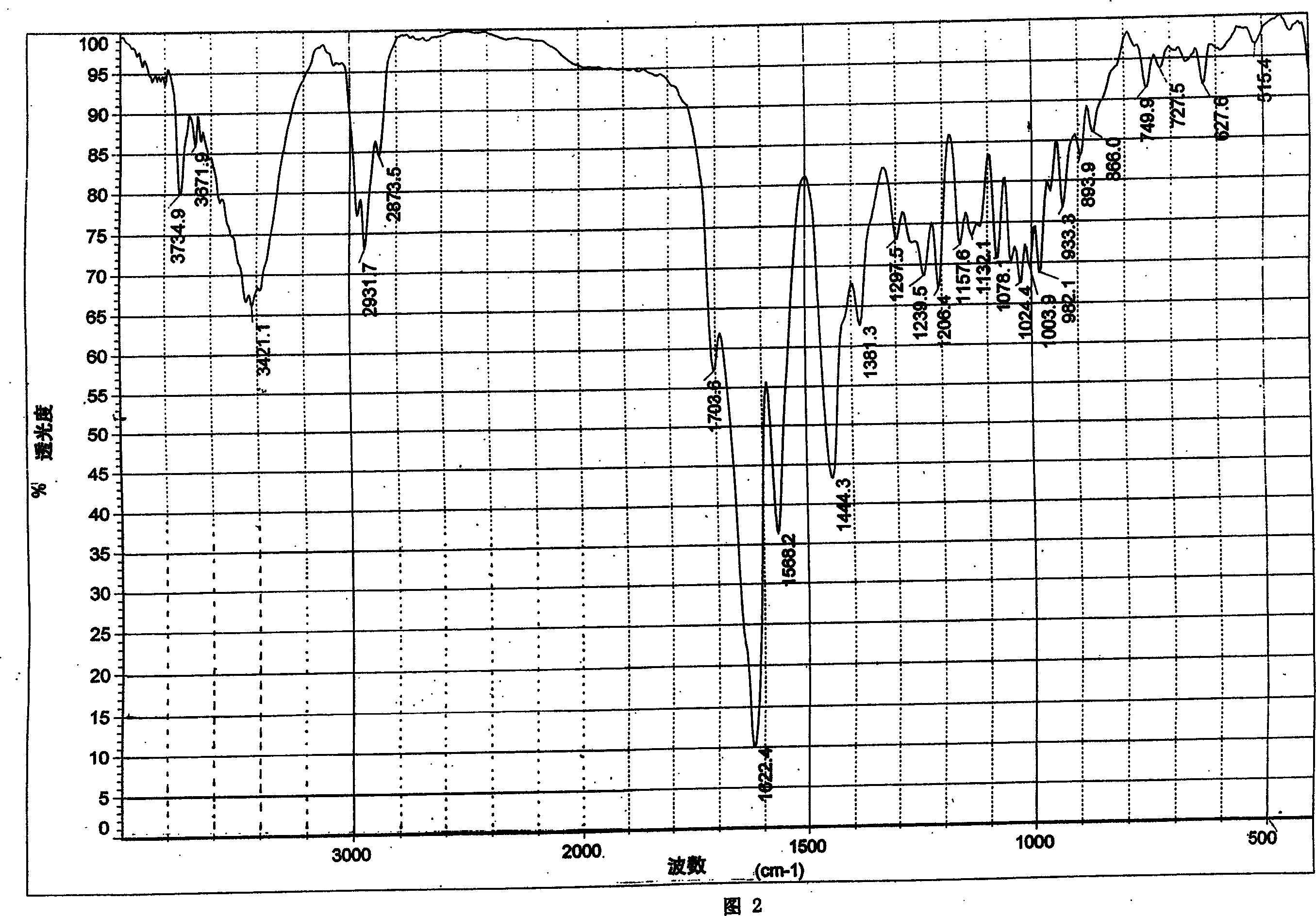
Image
Examples
Embodiment 1
[0029] Preparation of compound (I)
[0030] (1) Strain cultivation and fermentation:
[0031] ①Preparation of culture medium (g / l): Take 5.0g of glucose, 40.0g of soluble starch, 4.0g of peptone, 2.0ml of corn steep liquor, K 2 HPO 4 0.5g, MgSO 4 ·7H 2 O 0.5g, NaCl 0.5g, add water to 1000ml at pH 6.0, sterilize at 121°C for 15min; ②Fermentation: add Streptomyces luteogriseus via. In the sterilized medium, the amount of bacteria added is 10% of the weight of the fermentation medium, and the shake flask is fermented at 160 rpm, 28°C, and fermented for 4 days;
[0032] (2) Fermentation broth treatment: ①The fermentation broth was centrifuged at 4800 rpm for 20 minutes; ②Extracted 5 times with an equal volume of ethyl acetate, and the ethyl acetate part was back-extracted with 2:1 volume of water to remove pigment impurities; ③Recover ethyl acetate to obtain a brown oil with an active site; ④Carry out silica gel column chromatography, use dichloromethane:methanol 1:0 to 0:1 ...
Embodiment 2
[0049] Determination of antibacterial activity of the compound prepared in Example 1 against Bacillus subtilis. Bacillus subtilis was used as the test bacteria, and the following positive control drugs were selected:
[0050] Erythromycin (Tianjin Jinshi Pharmaceutical Co., Ltd., batch number: 020302);
[0051] Acetylspiramycin (Tianjin Zhongxin Pharmaceutical Group Co., Ltd. Xinxin Pharmaceutical Factory, batch number: 030104);
[0052] Lijunsha (erythromycin ethylsuccinate, Xi'an Lijun Pharmaceutical Co., Ltd., batch number: 0303075-14);
[0053] According to the Oxford cup and saucer method, the agar gel was spread on a plane, and after 24 hours, the diameter of the inhibition zone was measured.
[0054] Adopt the concentration that same wants to compare sample (2 mg / ml) and the compound (2 mg / ml) prepared by the embodiment of the present invention 1, survey its bacteriostatic zone, the bacteriostatic zone of compound of the present invention is far greater than that of p...
Embodiment 3
[0056] The compound prepared in Example 1 is also effective against drug-resistant Staphylococcus aureus.
[0057] The minimum inhibitory concentration (MIC) for Staphylcoccus aureus (Staphylcoccus aureusATCC29213) is 12 μg / ml.
[0058] The minimum inhibitory concentration (MIC) for Roxithromycin-resistant Streptococcus pneumoniae (RRSP) is 0.5 μg / ml.
[0059] The above strains were all preserved by the Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com