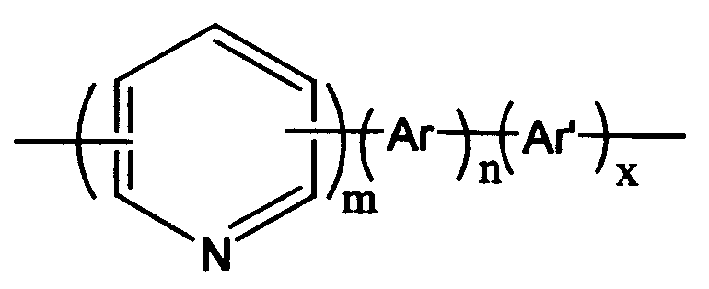Conjugated polymer containing pyridine on main chain, preparation process and use thereof
A technology of conjugated polymer and pyridine, which is applied to the conjugated polymer containing pyridine on the main chain and the fields of its preparation and use, can solve the problem that polypyridine cannot transport holes well, pollute the environment, and the luminescence performance of polymers, single The problem of low efficiency of layer light-emitting devices, etc., achieves the effects of low cost, changing the light-emitting wavelength, and easy industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1: A 100 ml three-necked reaction flask is equipped with a water condenser, a constant pressure dropping funnel, and an argon gas introduction tube. The argon gas is led out from the top of the condenser tube, and connected to the atmosphere through a drying tower filled with calcium chloride or sodium hydroxide. A stirring magnet is placed in the bottle, and the reaction bottle is placed on the magnetic stirrer. Before the reaction, pass argon gas for five minutes. Under the protection of argon flow, add 0.38 g (4 mmol) of pyridine N-oxide and 20 ml of chloroform to dissolve the pyridine oxide, then add 3.9 g (24 mmol) of anhydrous ferric trichloride, and keep Add 0.5 g (2 mmol) of N-hexylcarbazole into the pressure dropping funnel and dissolve in 15 ml of chloroform, slowly add N-hexylcarbazole dropwise into the three-necked flask, stir with magnetic force, and react at 28°C for 40 hours. Then the reaction solution was poured into 100ml ethanol, stirred for t...
Embodiment 2
[0014] Embodiment 2: The device used is the same as in Example 1. Add 0.67 g (7 mmol) of pyridine N-oxide and 25 ml of chloroform into the three-necked flask, and after dissolving, add 6.8 g (42 mmol) of anhydrous ferric chloride. Add 0.86 g (3.5 mmol) of triphenylamine and 15 ml of chloroform into the constant pressure dropping funnel. Slowly add triphenylamine dropwise, react at 40°C for 36 hours, pour into 120ml of ethanol, stir for 2 hours, and filter with suction to obtain a conjugated polymer of yellow precipitated pyridine oxide and triphenylamine, which is extracted with ethanol after drying. After drying (50° C.), the weight was 0.72 g, and the yield was 47.1%. Reduction: Dissolve 0.31g of the obtained product in 20ml of frozen chloroform, add 0.4ml of phosphorus trichloride, control the temperature at 75°C, react for one hour and then cool, then add 20ml of water and stir, neutralize with sodium hydroxide until alkaline, Collect the organic phase in the reaction bo...
Embodiment 3
[0015] Example 3: A 100 ml three-necked reaction flask is equipped with a water condenser and an argon inlet tube. The argon gas is led out from the top of the condenser tube, and connected to the atmosphere through a drying tower filled with calcium chloride. A stirring magnet is placed in the bottle, and the reaction bottle is placed on the magnetic stirrer. Before the reaction, pass argon for five minutes. Under the protection of argon flow, add 0.57 g (6 mmol) of pyridine N-oxide and 0.51 g (3 mmol) of fluorene from another opening of the three-neck flask (stopped with a ground stopper before this), 30ml of chloroform was dissolved, then 5.85g (36mmol) of anhydrous ferric trichloride was added, reacted in an ice-salt bath for 48 hours, poured into 100ml of ethanol, stirred for 2 hours, and suction filtered to obtain yellow solid pyridine oxide and The conjugated polymer of fluorene was dried in the air, extracted with ethanol, dried (50° C.), weighed: 0.68 g, and the yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com