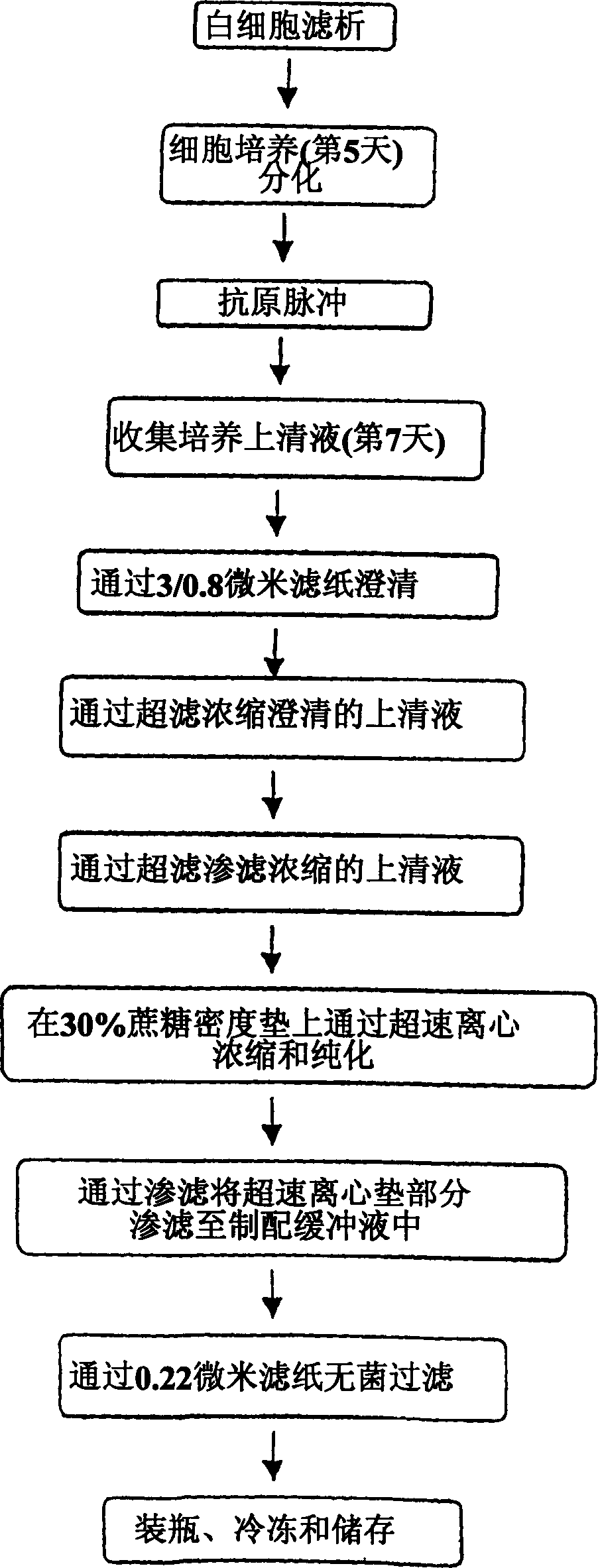
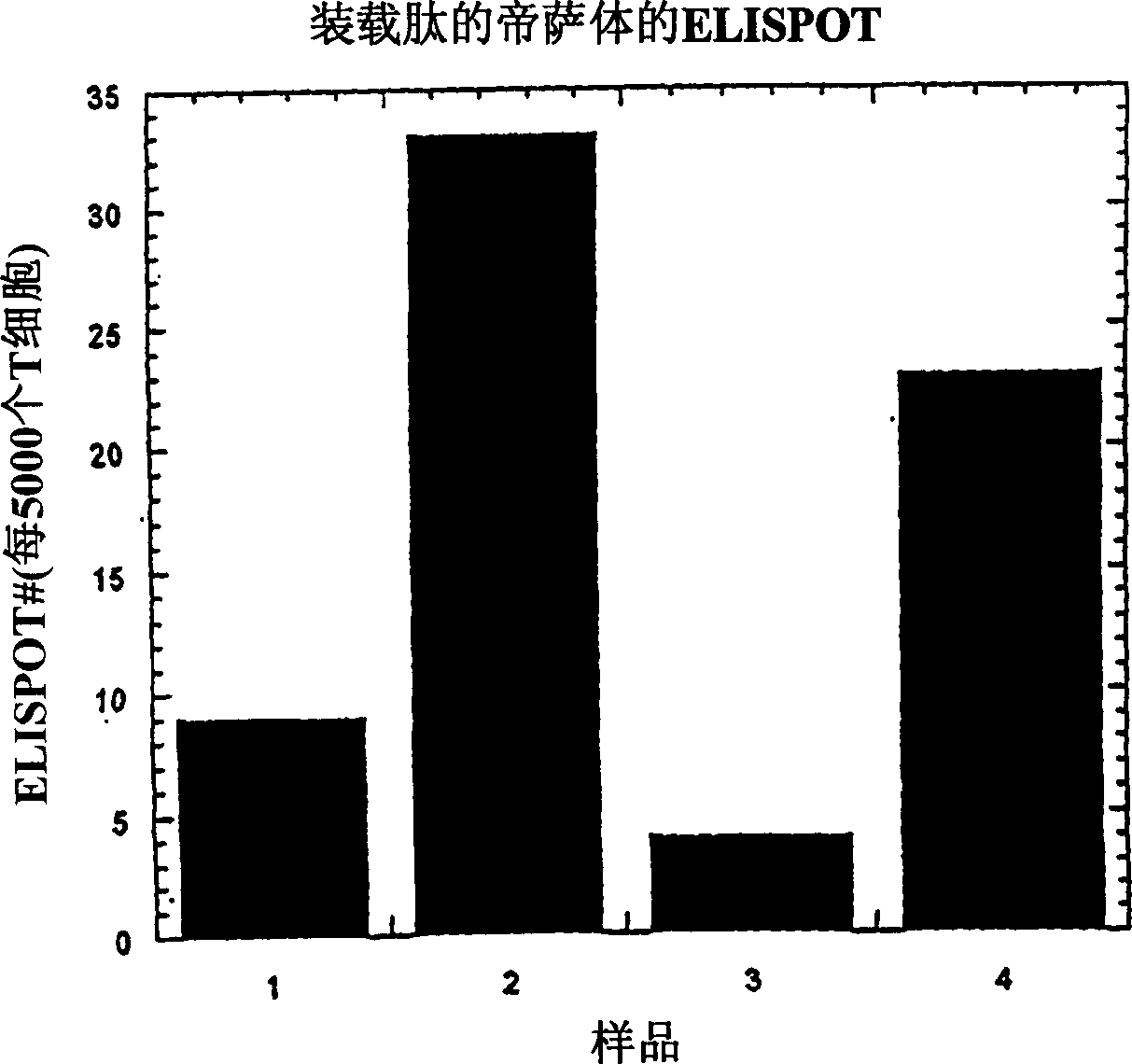
Method of producing membrane vesicles
A technology of membrane capsules and purification membranes, applied in biochemical equipment and methods, chemical instruments and methods, microorganisms, etc., can solve the problems of cell debris pollution, low recovery, poor reproducibility of operator changes, etc., and achieve high occupancy, Effect of increasing immunogenic potential, increasing efficiency of direct loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0201] In the preparation method of the present invention, other techniques, such as ion exchange and affinity chromatography and flow field-flow fractionation, can also be used to concentrate Tissa body. percolation
[0202] Diafiltration can be used on concentrated Tissa system preparations to reduce the concentration of contaminating media and cellular proteins. Diafiltration is performed according to several techniques that allow exchange of sample buffer with formulated buffer, including ultrafiltration, chromatography, ultracentrifugation, and through dialysis bags.
[0203] In a preferred embodiment, diafiltration is performed using an ultrafiltration system. This particular embodiment is effective as demonstrated in the experimental section. Furthermore, if the capsule preparation has been concentrated by ultrafiltration, the diafiltration steps can easily be combined using the same methodology.
[0204] In this regard, in certain embodiments, exosomes are diafilte...
Embodiment 1
[0265] Other aspects and advantages of the present invention are shown in the following examples, which should be considered as illustrations and not as limitations of the invention. Embodiment 1. Preparation of culture medium
[0266] Prior to use, the medium has been treated by ultrafiltration (in this example, clinical grade AIM V is a serum-free cell culture medium from Life Technologies, Inc.) in order to remove clumped proteins, removal of clumped proteins It does not affect cell growth, but considerably contributes to the subsequent isolation of pure Disa bodies.
[0267] Ultrafiltration of the medium was performed using a 500 kDa hollow fiber membrane (from A / G Technology, NeeDham, Mass or from a vendor with related products). The size of the membrane retains protein aggregates in the retentate while allowing non-aggregated proteins to pass through with the filtrate. The filtrate was collected, sterile filtered through 0.22 micron filter paper into vials, and stored ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com