Carbon negative electrode material of lithium ion cell, its preparation method and application
A technology for lithium ion batteries and negative electrode materials, which is applied in electrode manufacturing, battery electrodes, circuits, etc., can solve the problems of low first charge and discharge efficiency, poor cycle performance, etc., to improve the first charge and discharge efficiency, reduce the degree of anisotropy, Reduce the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
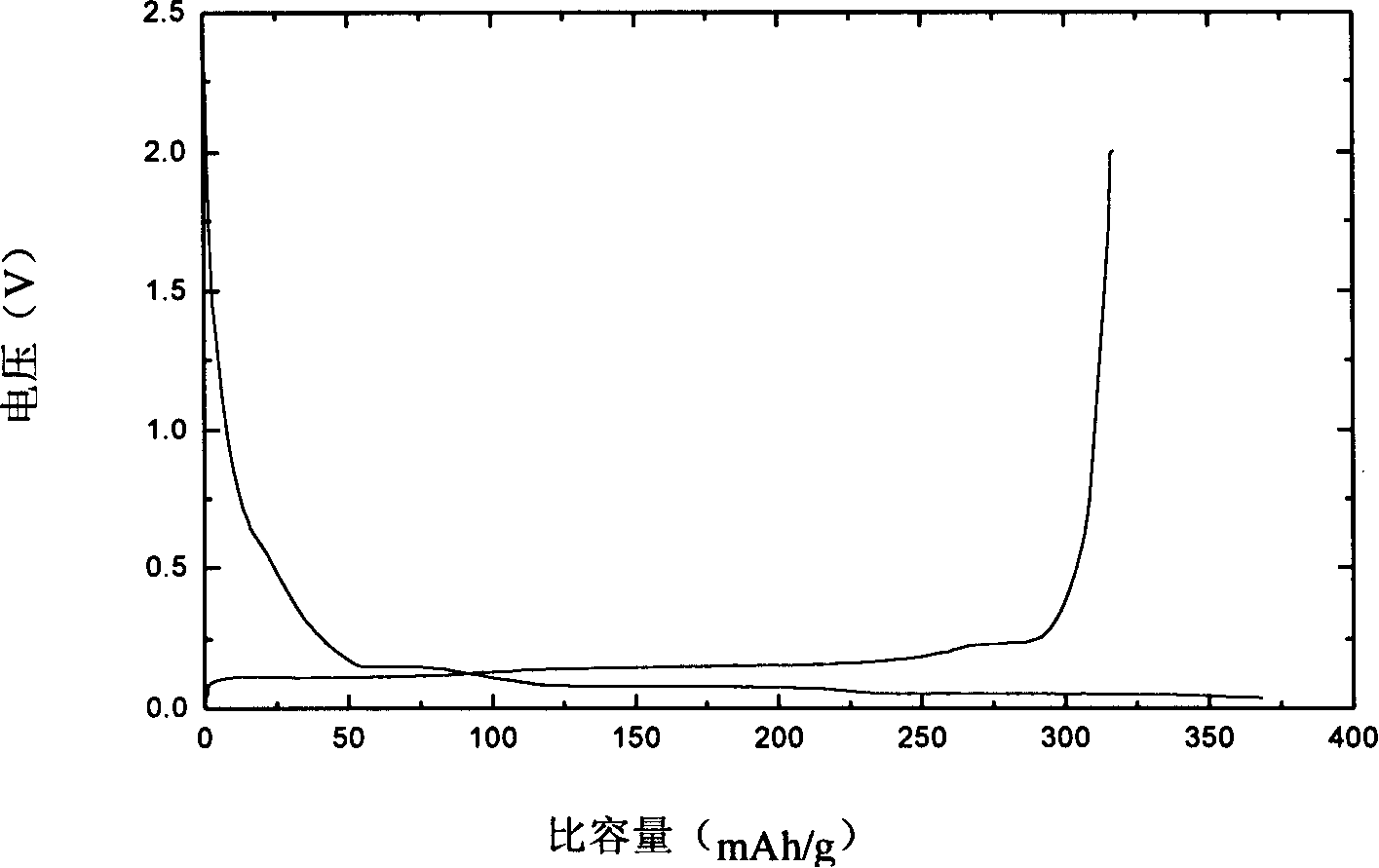
[0021] Take by weighing 20 grams of natural graphite and add the concentration to be 20% HClO 4 Fully stirred for 5 hours, the reaction temperature was 45°C, and then filtered and dried. Add 0.25 gram polystyrene sulfonate lithium (molecular weight 6500) in natural graphite after above-mentioned treatment, 34 milliliters of methanol solvents are made dispersion medium, transfer in the radiant tube after fully stirring, obtain surface adsorption has polystyrene sulfonate lithium graphite material. The above-mentioned graphite material was placed in a cobalt 60-γ-ray radiation source for cross-linking polymerization, the reaction time was 16 hours, the dose was 1.6 million rad, and the dose rate was 100,000 rad / h. Afterwards, it was washed three times with anhydrous methanol to obtain a polymer solid electrolyte-coated graphite material. The above materials were made into electrode sheets with a thickness of 0.1-0.2 mm and assembled into batteries to perform charge and dischar...
Embodiment 2
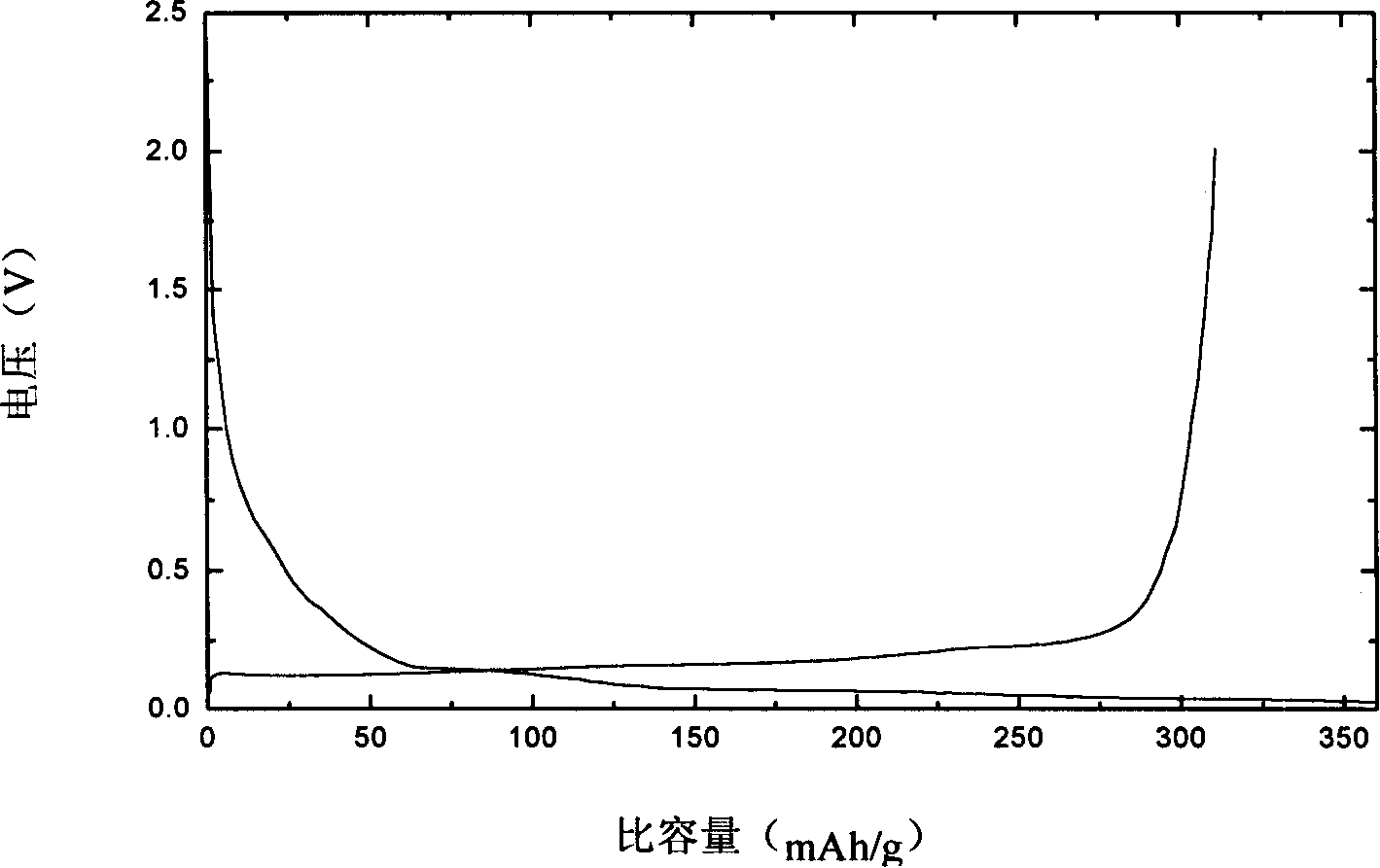
[0023] Weigh 20 grams of natural graphite and add a concentration of 30% H 2 SO 4 Fully stirred for 10 hours, the reaction temperature was 35°C, and then filtered and dried. Add 0.15 gram polystyrene sulfonate lithium (molecular weight 4000) in natural graphite after above-mentioned treatment, 34 milliliters of ethanol solvents are made dispersion medium, transfer in the radiant tube after fully stirring, obtain surface adsorption has polystyrene sulfonate lithium graphite material. The above-mentioned graphite material was placed in an electron accelerator for cross-linking polymerization, the reaction time was 20 hours, the dose was 2.4 million rad, and the dose rate was 120,000 rad / h. Afterwards, it was washed three times with anhydrous methanol to obtain a polymer solid electrolyte-coated graphite material. The above materials were made into electrode sheets with a thickness of 0.1-0.2 mm and assembled into batteries to perform charge and discharge performance tests.
Embodiment 3
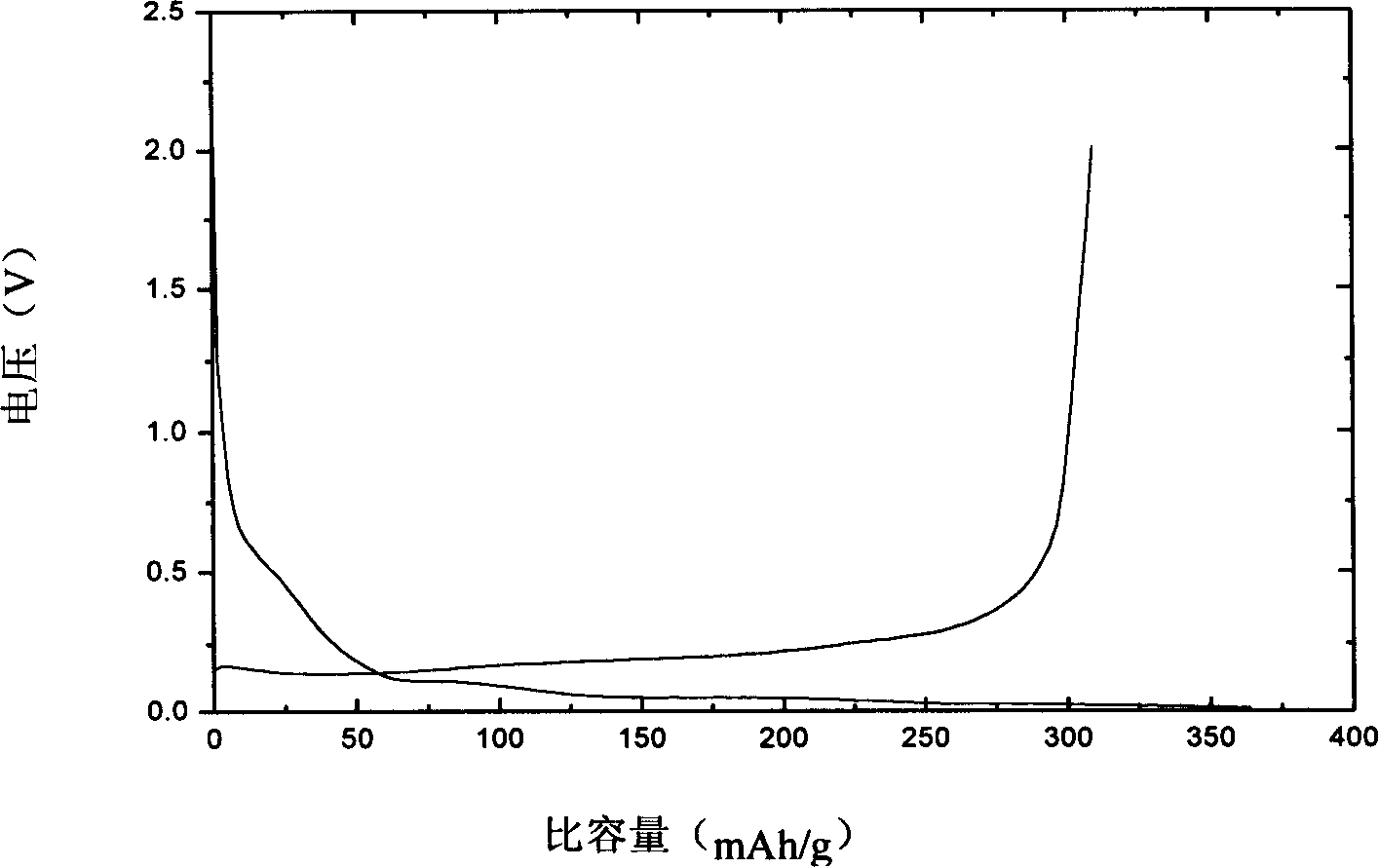
[0025] Weigh 20 grams of natural graphite and add concentration of 40% HNO 3 Fully stir in the medium for 8 hours, the reaction temperature is 50°C, and then filter and dry. Add 0.20 grams of methacrylate oxide ethylene ester and lithium methacrylate monomer with a weight ratio of 1:0.5 to the natural graphite after the above treatment, and 34 milliliters of a volume ratio of 1:0.6 water / ethanol solution as a dispersion medium, transfer after fully stirring In the radiant tube, a graphite material with monomers adsorbed on the surface is obtained. The above-mentioned graphite material was placed in a cobalt 60-γ-ray radiation source for copolymerization reaction, the reaction time was 16 hours, the dose was 3.2 million rad, and the dose rate was 200,000 rad / h. Afterwards, it was washed three times with anhydrous methanol to obtain a polymer solid electrolyte-coated graphite material. The above materials were made into electrode sheets with a thickness of 0.1-0.2 mm and assem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com