Stabilized denatured lipoprotein and process for producing the same
A lipoprotein and stabilization technology, applied in the field of preparation of denatured lipoprotein, stabilized denatured lipoprotein and its preparation, can solve the problems of unsatisfactory storage stability and cumbersome operation of standard substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 4 approach
[0057] According to the fourth embodiment of the present invention, the present invention provides the stabilized denatured lipoprotein prepared by the above-mentioned first or third embodiment.
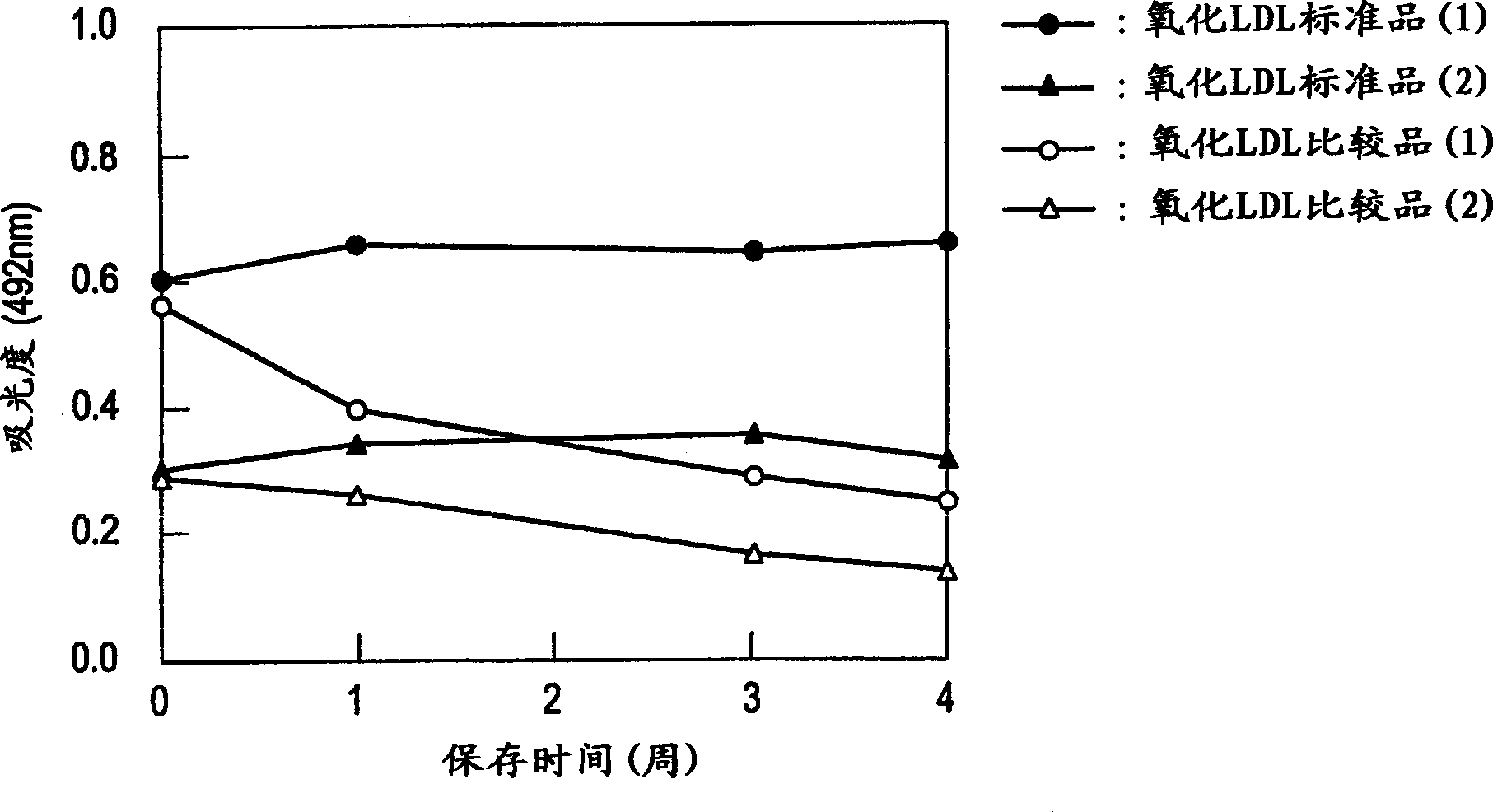
[0058] The denatured lipoprotein and the stabilized denatured lipoprotein prepared by the above steps have excellent long-term storage stability, for example, they are brought into contact with an antibody that recognizes the denatured lipoprotein, and the reactivity of the antibody to the sample is measured to measure It can be used as a standard substance in methods containing denatured lipoproteins, and can also be used as various experimental reagents for studying the physiological functions and activities of denatured lipoproteins.
no. 5 approach
[0059] Therefore, according to the fifth embodiment of the present invention, the present invention provides a method for measuring denatured lipoprotein, which method uses the stabilized denatured lipoprotein prepared by the above-mentioned first or third embodiment as a standard substance. In addition, according to the sixth embodiment of the present invention, the present invention provides a kit for measuring denatured lipoprotein, which kit contains the stabilized denatured lipoprotein prepared by the above-mentioned first or third embodiment as a standard substance.
[0060] In the above-mentioned fifth embodiment, the method of measuring denatured lipoprotein is not particularly limited, and a known method can be used. Specifically, denatured lipoproteins are brought into contact with antibodies that recognize denatured lipoproteins, and the reactivity of the antibodies to a sample is measured to measure denatured lipoproteins contained in blood components. For example, ...
Embodiment 1
[0065] (1) Preparation of LDL
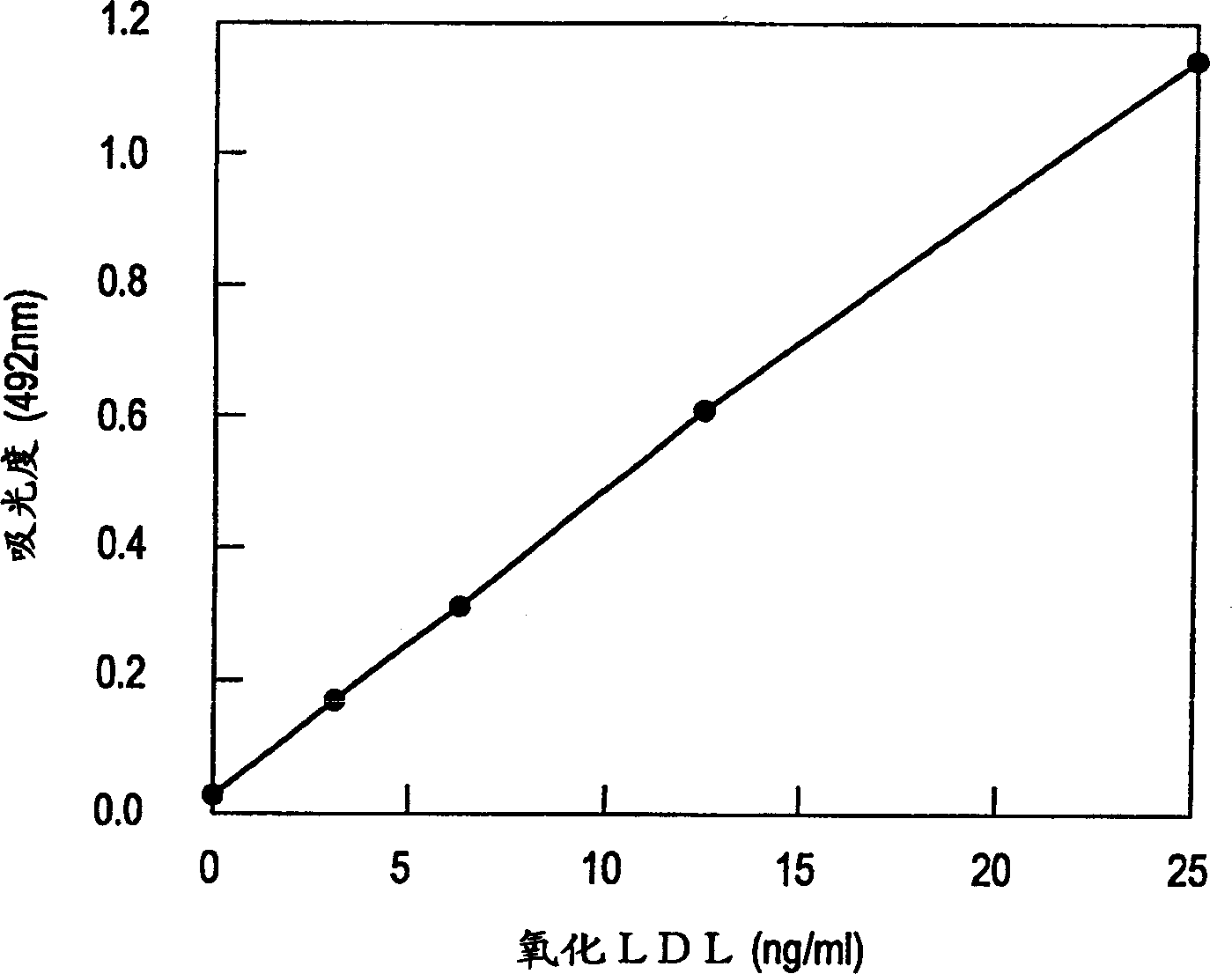
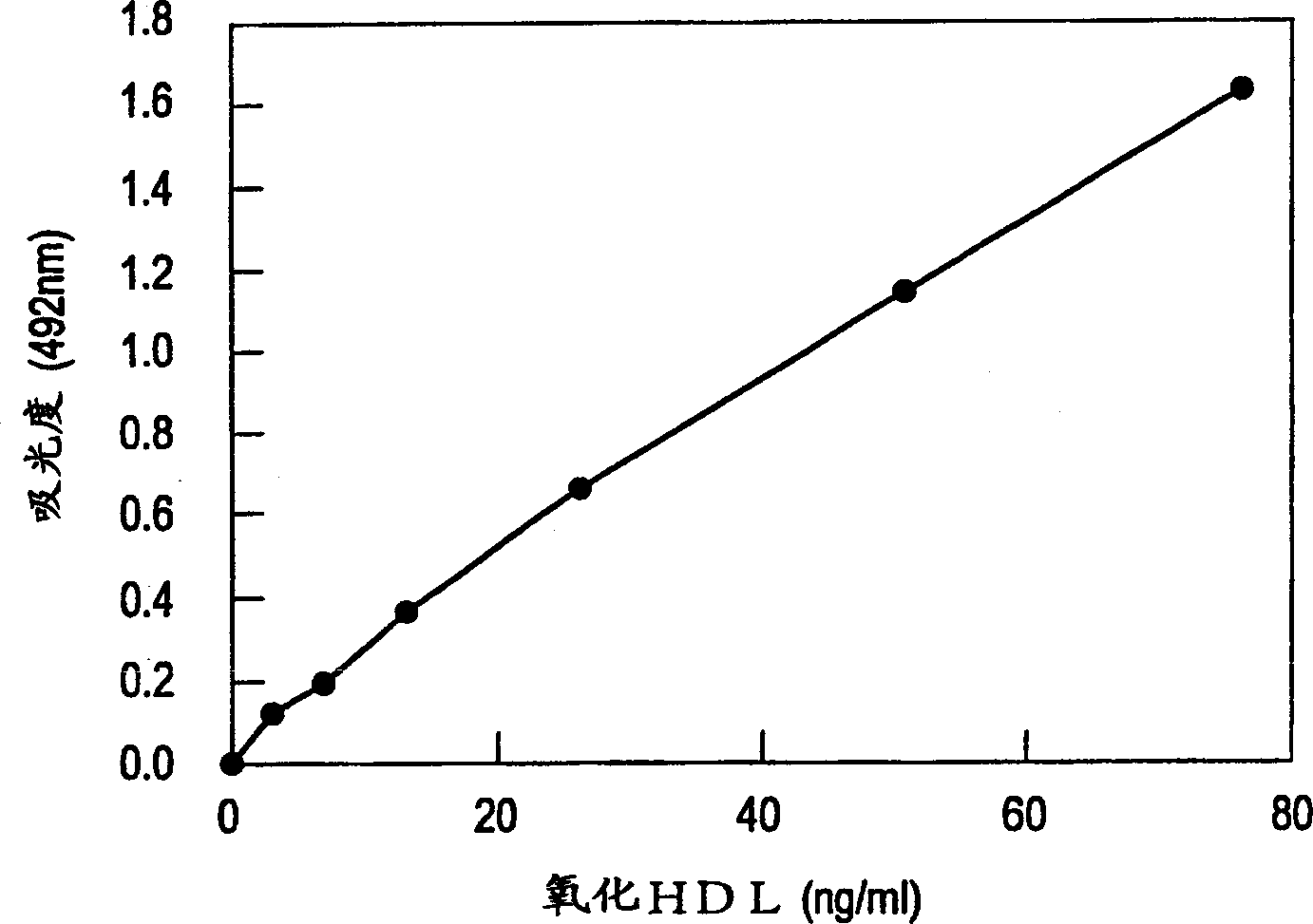
[0066]Obtain ultracentrifugation from human plasma obtained with EDTA as an anticoagulant (10°C, adjust the specific gravity to 1.019, 120000×g, centrifuge for 20 hours, recover the upper layer, adjust the specific gravity to 1.063, and centrifuge at 120000×g for 24 hours) The portion with a specific gravity of 1.019 to 1.063 is recovered as an LDL component. At this time, the purity of LDL was confirmed by obtaining a single sharp band in agarose gel electrophoresis.
[0067] Next, the LDL fraction was purified by sufficient dialysis (16 hours or overnight) against PBS (pH 7.4) containing 0.25 mmol / L EDTA. The above-mentioned purified LDL was then dissolved in PBS (pH 7.4) containing 0.25 mmol / L EDTA to make the LDL protein concentration 1 mg / mL, and stored at 4°C for future use. In this example, the protein content was determined by Lowry's improved method. Briefly, 2(w / v)% sodium carbonate, 0.4(w / v)% sodium hydroxide, 0.16(w / v)% tartaric a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com